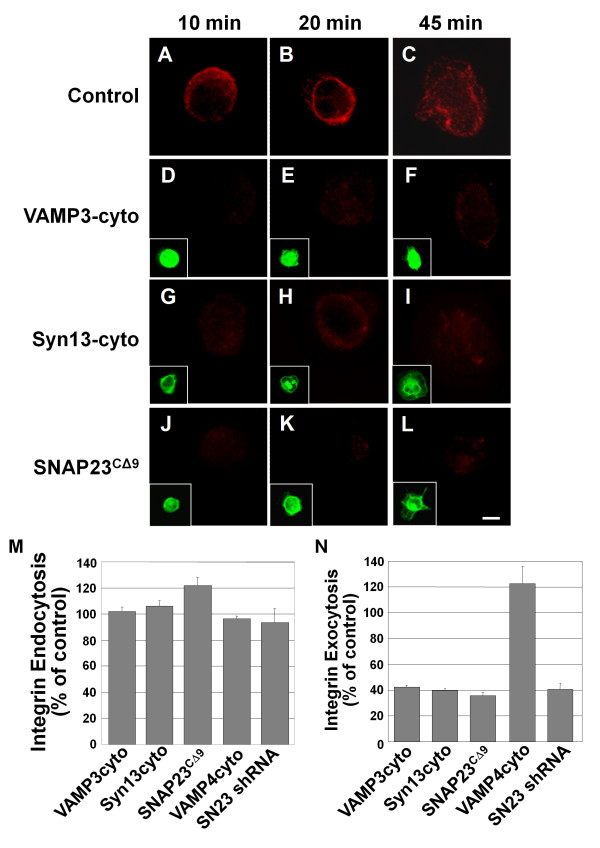
Figure 4.
Dominant-negative SNARE domains inhibit β1 integrin exocytosis. CHO-K1 cells were transiently transfected for 21 h with GFP-tagged constructs of truncated syntaxin13 (Syn13cyto)[D-F], VAMP3 (VAMP3cyto)[G-I], VAMP4 (VAMP4cyto) or SNAP23 (SNAP23CΔ9 - 15 h transfection) [J-L]. Untransfected control cells are shown in A-C. HeLa cells were transfected with a SNAP23 shRNA construct (SN23 shRNA) for 72 h. Cells were incubated with β1 integrin antibody in serum free media for 3 h to allowing internalization of the label. Then the cells were lifted with trypsin, removing remaining surface label, and were plated on 20 μg/mL FN for 10 min (A, D, G, J,), 20 min (B, E, H, K,) or 45 min (C, F, I, L). For exocytosis assays, cells were fixed and any surface exposed labeled-integrin was stained with AlexaFluor 594 secondary antibody (A-L, data for 20 min of adhesion shown in N). Insets show expression of GFP-tagged constructs (D-L). Images are 3 D reconstructions of a z-series. Scale bar represents 10 μm. (M and N) Integrin immunofluorescent staining at 20 min. was quantified in micrographs using ImageJ software. (M) To assess endocytosis of labeled integrin, cells were permeablized with 0.2% Trition X-100 in PBS before staining with AlexaFluor 594 secondary antibody (percent of non-transfected control). (N) Labeled integrin detected on the cell surface in non-permeabilized cells (percent of non-transfected control). Means ± SEM from three independent experiments (at least 20 cells per experiment) are shown in M and N.