Abstract
Mutations and knockout of aquaporin 0 (AQP0) result in dominant lens cataract. To date, several functions have been proposed for AQP0; however, two functions, water permeability and cell-to-cell adhesion have been supported by several investigators and only water channel function has been readily authenticated by in vitro and ex vivo studies. Lens shifts protein expression from the more efficient AQP1 in the equatorial epithelial cells to the less efficient water channel, AQP0, in the differentiating secondary fiber cells; perhaps, AQP0 performs a distinctive function. If AQP0 has only water permeability function, can the more efficient water channel AQP1 transgenically expressed in the fiber cells compensate and restore lens transparency in the AQP0 knockout (AQP0-/-) mouse? To investigate, we generated a transgenic wild type mouse line expressing AQP1 in the fiber cells using αA-crystallin promoter. These transgenic mice (TgAQP1+/+) showed increase in fiber cell membrane water permeability without any morphological, anatomical or physiological defects compared to the wild type indicating that the main purpose of the shift in expression from AQP1 to AQP0 may not be to lessen the membrane water permeability. Further, we transgenically expressed AQP1 in the lens fiber cells of AQP0 knockout mouse (TgAQP1+/+/AQP0-/-) to determine whether AQP1 could restore AQP0 water channel function and regain lens transparency. Fiber cells of these mice showed 2.6 times more water permeability than the wild type. Transgene AQP1 reduced the severity of lens cataract and prevented dramatic acceleration of cataractogenesis. However, lens fiber cells showed deformities and lack of compact cellular architecture. Loss of lens transparency due to the absence of AQP0 was not completely restored indicating an additional function for AQP0. In vitro studies showed that AQP0 is capable of cell-to-cell adhesion while AQP1 is not. To our knowledge, this is the first report which uses an animal model to demonstrate that AQP0 may have an additional function, possibly cell-to-cell adhesion.
Keywords: AQP0, AQP1, AQP0 knockout mouse, lens cataract, transgenic mouse, αA-crystallin promoter, lens, water permeability
1. Introduction
The vertebrate lens is a transparent tissue that lacks blood vasculature and grows continually throughout life with the oldest embryonic primary fiber cells occupying the center and the youngest secondary fiber cells forming the outermost layer. Mammalian lens consists of two types of cells of ectodermal origin, a monolayer of epithelial cells on the anterior surface and elongated fiber cells in the core of the lens. Aquaporins (AQPs) are transmembrane channels that facilitate passive movement of water across the plasma membrane. Two structurally similar aquaporins, AQP0 (or MIP26) and AQP1, are expressed in the lens (see Supplementary data, Fig. S1, A-C) and play a significant role in lens transparency and homeostasis (Chepelinsky, 2009; Mathias et al., 2007; Shiels et al., 2001; Varadaraj et al., 1999, 2005, 2008). AQP1 is expressed in the epithelial cells and its expression is down-regulated and replaced by AQP0 during fiber cell differentiation from the equatorial epithelial cells. AQP0 is abundant in the lens fiber cells, constituting over 50% of the total membrane protein thus making the fiber cell membrane considerably water permeable. During accommodation, the lens gains or loses about 2-8% of its volume for becoming rounder or more flat and the in and out fluid movement for this process must be through AQP0 in the fiber cells; aging-related loss of accommodative power is caused by alterations in the fluid conductance by AQP0 in the lens fiber cells (Gerometta et al., 2007). Low, high or no expression of aquaporins in mammals results in water imbalance (Detmers et al., 2006).
AQP1 knockout and mutation in mice as well as mutation in humans did not lead to cataract under normal conditions. However, AQP1 deficiency induced cataract in vitro and in vivo in mouse lenses under stressful conditions (Ruiz-Ederra and Verkman, 2006). Yet, there is no report in literature on lens cataract development under stressful conditions in humans with AQP1 mutation. On the contrary, autosomal dominant lens cataract develops due to loss or mutation of AQP0 in mice (Al-Ghoul et al., 2003; Okamura et al., 2003; Shiels and Bassnett, 1996; Shiels et al., 2001; Varadaraj et al., 1999) and due to mutation of AQP0 in humans (Francis et al., 2000; Geyer et al., 2006; Gu et al., 2007; Lin et al., 2007; Varadaraj et al., 2008) indicating the indispensable role of AQP0 for lens transparency. We have shown that AQP0 is responsible for ∼80% of the water permeability in mouse lens fiber cell plasma membranes (Shiels et al., 2001; Varadaraj et al., 1999). AQP0 knockout and mutant mice show loss of membrane water permeability in a dose dependent manner. Loss of AQP0 is further associated with a significant reduction in the focusing ability (Shiels et al., 2001). Loss of even a single copy of AQP0 affects the size of the lens due to disintegration of the fiber cells stemming from the loss of homeostasis. Mutation of AQP0 induces cytotoxicity as a consequence of the cytoplasmic accumulation of mutant protein causing fiber cell swelling and disintegration; cytotoxicity leads to microphthalmia (Varadaraj et al., 2008).
Even though AQP0 had been experimentally proven to provide water permeability to the fiber cell membranes, this function does not address the necessity for the lens to switch protein expression from AQP1 in the anterior epithelial cells to a much less efficient water channel, AQP0 in the secondary fiber cells that differentiate from the equatorial epithelial cells. We hypothesize that besides being a water channel, AQP0 may perform a unique function. Tissue-specific expression of a closely related aquaporin provides the perfect model to study the purpose of water channel diversity with regard to lens transparency. Using an Enhanced Green Fluorescent Protein (EGFP) tagged AQP1 transgenic mouse model and an AQP0 knockout (AQP0-/-) mouse model, we developed a transgenic model expressing human AQP1 (which is normally expressed in the epithelial cells) in the fiber cells of AQP0 knockout mouse (using mouse αA-crystallin promoter) to study the role of AQP0 in lens transparency and homeostasis. AQP1 and AQP0 are expressed in the lens, have similar topology and perform water channel function in the lens (Ball et al., 2003; Chandy et al., 1997; Kushmerick et al., 1995; Mulders et al., 1995; Nemeth-Cahalan and Hall, 2000; Shiels et al., 2001; Varadaraj et al., 1999, 2007, 2005, 2008; Zampighi et al., 1995). Given the compatibilities of the two aquaporins, AQP1 may be able to fulfill the water channel function of AQP0 in the lens fiber cells, restore homeostasis and prevent lens cataract development in AQP0 knockout (AQP0-/-) mouse model if lens transparency is due to AQP0 water channel function. Conversely, if AQP0 has a unique role, AQP1 may not be able to fully replace AQP0 functionally. This is the first study seeking to elucidate the functional role of AQP0 using a gene substitution method.
Our study demonstrates that the transgenically expressed, highly efficient water channel AQP1 was able to restore only partial lens transparency in the cataractous lenses of AQP0-/- mouse and suggests that AQP0 performs at least one unique function that cannot be replaced by AQP1.
2. Materials and Methods
2.1. Generation and Characterization of Transgenic AQP1 Mice
Construction of human AQP1-EGFP transgene is described in detail in the Supplemental Data section. A 2.2 kb DNA segment containing αA-crystallin promoter-AQP1-EGFP-SV40pA region was released from the vector sequences, gel purified and injected into the male pronucleus of single-cell embryos derived from inbred FVB mice (Charles River Inc.). Following standard methods (Hogan et al., 1994), the embryos were implanted into pseudopregnant CD1 females (Charles River Inc.) to produce transgenic founder mice (F0). Pups born were toe-marked and tail biopsies (1.5 mm) from 14 day-old pups were used for genotyping. These procedures were approved by the American Association for Accreditation of Laboratory Animal Care (AAALAC). Genomic DNA was extracted (Hogan et al., 1994) and PCR method (Varadaraj and Skinner, 1994) was used to detect the transgenics; a sense primer (prim1) to the murine αA-crystallin promoter and an antisense primer (prim5) to EGFP vector were designed for the purpose (Fig. 1A; Supplemental data, Table S1). The PCR products were fractionated on an agarose gel for analysis.
Fig. 1.
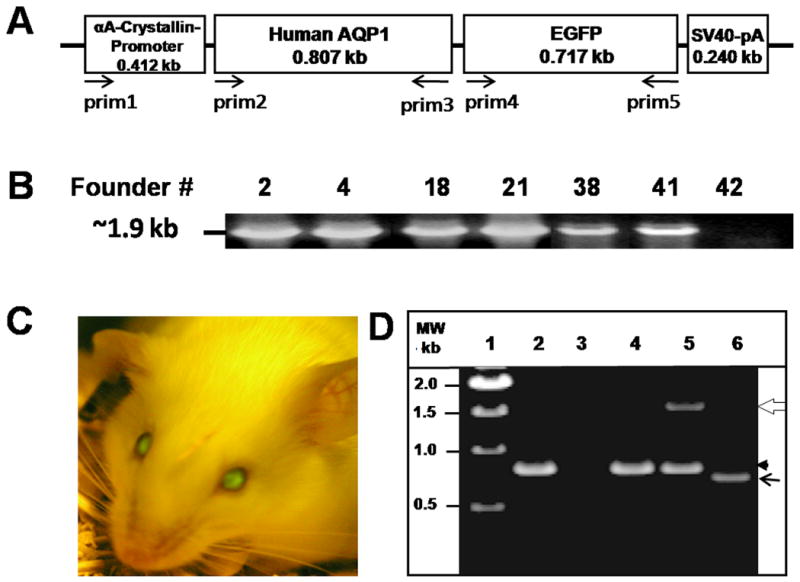
Generation and characterization of transgenic AQP1 (TgAQP1) mouse. A. Physical map of the construct used for generating AQP1-EGFP transgenic mice. Lens fiber cell-specific transgene construct containing αA-crystallin promoter, the coding region of human AQP1, an in-frame fluorescent epitope tag (EGFP) at the C-terminus of human AQP1 and SV40 polyA signal. Arrows indicate orientation and approximate location of the PCR primers. B. Genotyping to identify heterozygous transgenic founder mice. DNA from tail biopsies of pups (# 2, 4, 18, 21, 38, 41 and 42) was extracted and PCR method was used to detect the transgenics using sense (prim1) and anti-sense (prim5) primers. A band of 1.9 kb corresponding to the promoter + AQP1 + EGFP was amplified from the genomic DNA of each of the pups except for # 42. C. Identification of homozygous transgenic mouse (# 38) expressing EGFP in the lens, using Dark Reader. D. Examination of the expression of AQP1 and/or AQP1-EGFP transcripts in WT and TgAQP1+/+ lenses using RT-PCR. Lane 1, Molecular weight marker; lane 2, WT lens epithelial cells; lane 3, WT lens fiber cells; lane 4, TgAQP1+/+ epithelial cells; lanes 5 and 6, TgAQP1+/+ fiber cells. White open arrow, 1500 bp segment of AQP1-EGFP; black arrowhead, 800 bp segment of AQP1; black arrow, 700 bp segment of EGFP.
Using a special light and filter set, Dark Reader, expression of green fluorescence in the lenses of positive founders was identified. These founders were crossed to produce homozygous mouse expressing transgene AQP1 (TgAQP1+/+) in the fiber cells. We denote AQP1 transgenic mouse as TgAQP1 and heterozygous and homozygous transgenic mice with +/- and +/+ signs, respectively.
Recently, it has been reported (Simirskii et al., 2006) that FVB strain carries a CP49 mutation. Even though we did not test for the CP49 mutation, we monitored the lenses for abnormalities and cataracts for more than 25 generations; the lenses did not show any abnormalities, light scattering or cataract even after 5 years of breeding and rearing.
2.2. Generation of Homozygous TgAQP1+/+/AQP0-/- Mice
TgAQP1+/+ transgenic mice were paired with AQP0-/- to generate TgAQP1+/+/AQP0-/- mice. Since simple PCR-genotyping will not differentiate between heterozygous (TgAQP1+/-/AQP0+/-) and homozygous (TgAQP1+/+/AQP0-/-) animals, transgene positive offspring were crossed with wild type and the resulted offspring were genotyped, as well as observed for EGFP fluorescence using Dark Reader. To identify founders with autosomal integration of the AQP1 transgene, we have backcrossed the male or female TgAQP1+/+AQP0-/- with an appropriate mate from WT. Parents that produced all offspring with AQP1-EGFP expression in the lens were selected for further study. To confirm the expression of transgene AQP1 in the fiber cells of AQP0 knockout mice, PCR genotyping was performed using the following primers: prim1, 2, 3 and 5 (Supplemental data, Table S1). After identifying transgene zygosity and autosomal expression, the homozygous mice were inbred to set up a TgAQP1+/+AQP0-/- colony.
2.3. RT-PCR, Western Blotting and Immunostaining
These techniques were performed as described previously by Varadaraj et al. (1996, 1997, 2005, 2007) and Kumari et al. (2000, 2001). Details are given in the Supplemental Data section.
2.4. Lens Epithelial and Fiber Cell Membrane Water Permeability
Epithelial cells were isolated using collagenase. Total cortical region of the lens was separated from the lens nucleus and cortical fiber cell membrane vesicles were prepared. Water permeability of epithelial cells and fiber cell vesicles was calculated using the rate of shrinking and swelling method as described previously (Varadaraj et al., 1999). Digital video microscopy was used to estimate volume changes and membrane surface area. A cross-sectional image of each cell or vesicle was digitized and the area was determined using Sigma Scan Pro 5/ Image Analysis software.
2.5. Lens Focusing Ability and Transparency
Lens focusing ability and transparency of WT and TgAQP1+/+ mice were quantified by contrast ratio analysis of transmitted grid image versus transmitted non-grid image of the same lens using SigmaScan Pro5 software. Lens kept in a glass culture dish was placed on a copper metal grid (normally used for transmission electron microscopy; 400 mesh G400-Cu, Electron Microscopy Sciences, PA), illuminated from below and focused manually; light transmitted through the lens was imaged digitally using an Olympus C4040 zoom digital camera under the dark field. Image contrast ratios were quantified from a minimum of 5 images per lens and average was calculated. A minimum of 10 lenses each was analyzed from WT and TgAQP1+/+ mice for lens transparency as described by Varadaraj et al. (2007).
2.6. Light Microscopy
Eyes were dissected out and immediately transferred to petridishes containing prewarmed (37°C) lens physiological saline and placed on the microscopic stage set to 37°C. Lenses were dissected out, transferred to prewarmed glass culture dishes with lens physiological saline and photographed.
2.7. Fluorescent Microscopy
Dissection of lenses was carried out as described for light microscopy. Fluorescent images of AQP1-EGFP expressing lenses or immunostained lens sections with FITC conjugated secondary antibody were captured using Olympus CKX41 inverted fluorescent microscope equipped with Olympus C4040 zoom digital camera or Zeiss Axiovert 200 microscope equipped with AxioCam and Zeiss AxioVision 4.1 software (Carl Zeiss MicroImaging, Inc.).
2.8. Cell Aggregation Assay
Cell-to-cell aggregation assay using rotary gyratory shaker was performed as previously described (Kumari and Varadaraj, 2009). In brief, single cell suspensions of L-cells expressing empty vector (negative control), AQP0, AQP0-EGFP, AQP1, AQP1-EGFP, or E-cadherin (positive control) were each divided into two groups having fairly equal number of cells. One group was loaded with 5 μM/ml of CellTracker Red CMPTX dye. The other matching groups were loaded with CellTracker Blue CMAC (Invitrogen, CA) and mixed with the corresponding CellTracker Red CMPTX loaded cells in 1:1 ratio. Mixed cells were transferred to 6-well plates precoated with 2% agarose (Sigma) and rotated on a gyratory shaker (80 rpm) for 90 min, at 37 °C. At the end, equal volume of 2% glutaraldehyde was added to each well to prevent further aggregation or dissociation. Particle number (aggregates + individual cells) was determined using a hemocytometer. The extent of aggregation was calculated from the ratio of total particle numbers at time t of incubation (Nt) to the initial particle numbers (N0) and expressed as percentage. An aliquot of the experimental samples was imaged under an epifluorescent microscope.
3. Results
As a first step towards the production of the transgenic model, we engineered an AQP1-EGFP chimeric DNA and tested it in Xenopus oocytes as well as cultured cells to ascertain membrane localization and the effect of EGFP tag on the functional integrity of the construct. Next, we developed a transgenic wild type (WT) mouse line expressing the AQP1 transgene in the lens fiber cells. This step was considered because the physiological impact of the expression of a non-native and more efficient water channel (AQP1) could result in anomalous phenotypes due to increased water permeability and/or loss or gain of function/s. In the third and final step, we expressed the proven transgene construct in the fiber cells of AQP0 knockout mice and studied the outcome.
3.1. Functional Expression and Localization of AQP1 and AQP1-EGFP
Results for this section are given in detail in the Supplemental Data section. Overall, appending of the EGFP tag at the C-terminal end of AQP1 did not significantly alter the water channel function (Fig. S2 A-C), expression of the transcripts, trafficking or membrane localization of the protein (Figs. S2D and S3). These results strengthened the suitability of the AQP1-EGFP construct for generating a transgenic AQP1 (TgAQP1) model in the wild type mouse.
3.2. Generation of Homozygous Transgenic Wild Type Mouse Expressing AQP1-EGFP Transgene in the Lens Fiber Cells
AQP1 is a 28 kDa protein native to lens epithelial cells and AQP0 is a protein of similar size native to the lens fiber cells (Varadaraj et al., 2005, 2008). To gain a thorough understanding as to whether AQP0 performs more than one function, we sought to generate a transgenic (Tg) mouse model expressing a non-native aquaporin (AQP1) in the lens fiber cells. Mouse αA-crystallin gene promoter (Fig. 1A) was used to drive the transgene. Potential transgenic founder (F0) offspring were screened by isolating genomic DNA from tail biopsies and performing polymerase chain reaction (PCR). Genomic DNAs from fifty pups were subjected to PCR using primers prim1 and prim5 for amplification of a ∼1.9 kb fragment corresponding to the size of the complete αA-crystallin promoter-AQP1-EGFP transgene segment (Fig. 1A). Six positive pups or founders (# 2, 4, 18, 21, 38 and 41; Fig. 1B) carrying human AQP1-EGFP transgene with αA-crystallin promoter were identified by genotyping, and examining the eyes using a special light and filter set called Dark Reader for EGFP expression in the lens. These founder mice were crossed with WT FVB mice to generate F1 transgenics. One of the founders, #18 did not transmit the transgene to the F1 offspring. F2 mice were generated from intercross between the transgene-positive F1 offspring. F2 offspring were genotyped and as additional verification observed for EGFP expression in the lens. To determine zygosity, positive F2 offspring were backcrossed to WT FVB mouse. The resulting offspring were genotyped as well as observed for AQP1-EGFP fluorescence using Dark Reader. The F2 parents that produced all positive transgenic offspring were homozygous for AQP1 transgene (TgAQP1+/+) and were inbred to set up a homozygous colony. Lenses of F1 and F2 offspring from the founder #38 (Fig. 1C) were ∼2 times brighter than those from the offspring of other founders and selected for various analyses.
3.3. RT-PCR Analysis for AQP1-EGFP Transcripts in the Lens Fiber Cells
Samples of total RNA extracted from lens epithelial or fiber cells of genotyped transgenic mice were subjected to RT-PCR assay using two primer mixes, combining prim2, prim3 and prim5, or prim4 and prim5 (see Fig. 1A and Supplemental Data Table S1). Epithelial cell samples of WT and TgAQP1 mice reverse transcribed and amplified only a 0.8 kb DNA fragment (Fig. 1D, lanes 2,4) as expected using prim2, 3 and 5 primer mix revealing native AQP1 expression. Fiber cell RNA from WT mice showed no amplification (Fig. 1D, lane 3) with the same primer mix clarifying the natural absence of AQP1 transcripts since AQP1 is expressed only in the epithelial cells in the WT lenses. TgAQP1 lens fiber cell RNA amplified 0.8 and 1.5 kb segments as anticipated using prim2, 3 and 5 mix, indicating the presence as well as integrity of the transgene transcripts (Fig. 1D, lane 5). With prim4 and prim5, an amplicon of approximately 0.7 kb resulted (Fig. 1D, lane 6) corresponding to the EGFP portion (Fig. 1A) of the AQP1-EGFP mRNA transcript from the fiber cell RNA of the transgenics.
3.4. Onset and Continued Expression of Transgenic AQP1-EGFP Protein in the Embryonic and Adult Lenses of TgAQP1 mouse
In order to replace AQP0, the expression of transgenic AQP1 should commence at the time when AQP0 normally begins to express in the fiber cells. Immunohistochemical studies using anti-AQP1 antibodies revealed that AQP1-transgene began to express in the differentiating primary fiber cells of the embryonic lens at E11.25 (Fig. 2A, a). This was promising because it coincided with and reinforced our previous finding on the onset of AQP0 expression at stage E11.25 in the fiber cells of WT lens (Varadaraj et al., 2007). Transgene AQP1 continued to express in the fiber cells throughout the lens development (Fig. 2A, a-d), in postnatal stages (e.g., Fig. 2A, e) and in the adult (Fig. 2A, f; magnified outer cortex region). For comparison, a similar image of the WT mouse lens fiber cells expressing AQP0 is shown (Fig. 2A, g). The difference in the intensity of staining between Figure 2A (f) and (g) could be due to the difference in antibodies, cell types and/or levels of protein expression. Optical sections of adult TgAQP1+/+ and WT mouse lenses followed a similar pattern in antibody binding (Fig. 2B); there was an abrupt decrease in the intensity of antibody binding at the transition zone between differentiating and mature fibers, probably due to epitope masking. This result also verifies that transgene AQP1 had been post-translationally processed as the native AQP0 in the WT. Similar pattern of antibody binding was noticed in lens proteins like connexins and ion transporters like Na/K-ATPase. Originally it was speculated that the abrupt decrease in C-terminal antibody binding was due to post-translational C-terminal cleavage. Since antibodies raised against extracellular loop epitopes also failed to recognize the protein like the C-terminal antibodies, the abrupt decrease in antibody binding could be the result of post-translational modification-related epitope masking. In prenatal, postnatal and adult lenses of the transgenic mouse, primary and secondary fiber cells expressed AQP1 transgene protein in the membrane indicating proper folding and trafficking.
Fig. 2.
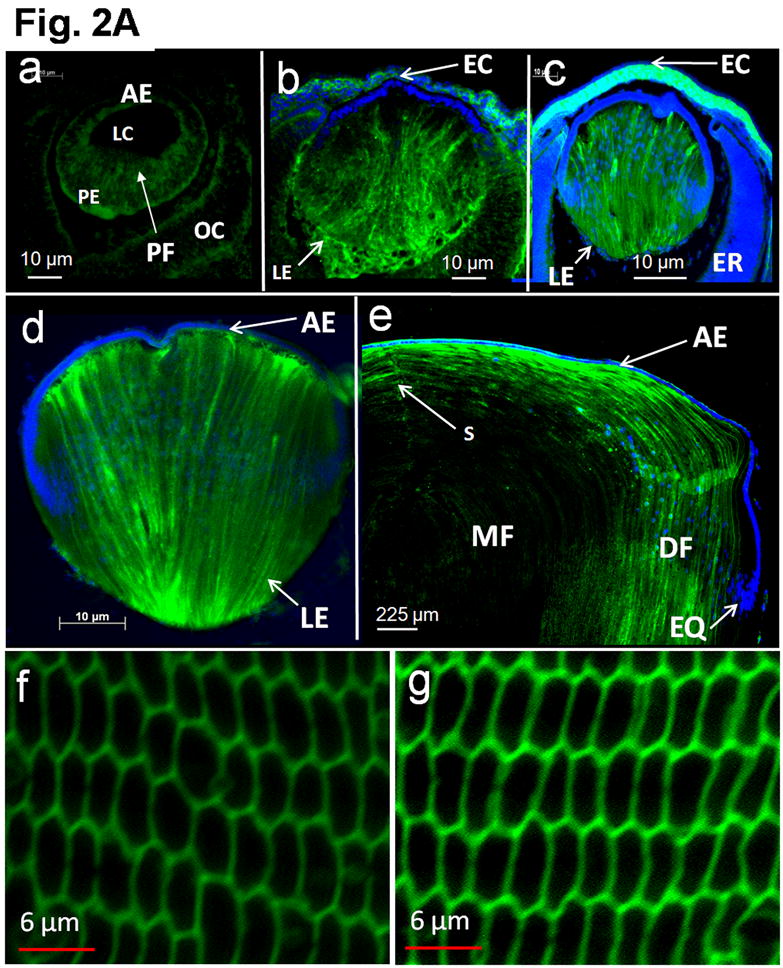
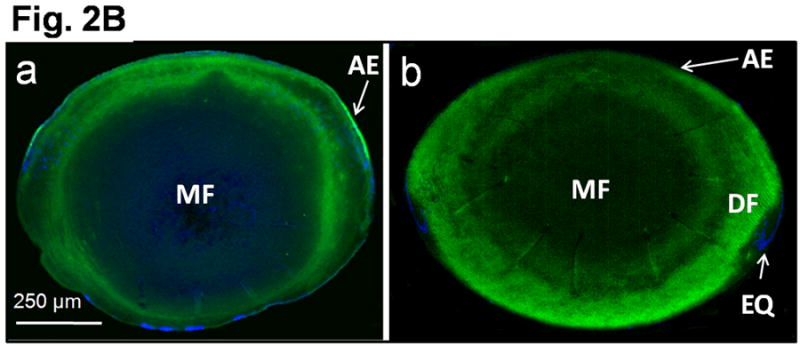
Transgene AQP1-EGFP expression in the developing mouse lens. A. Immunocytochemistry: Optical sections of TgAQP1+/+ eyes or lenses at: (a) E11.25; (b) E12.5; and (c) E15.5; (d) embryonic lens at E17.5 and (e) lens at postnatal day 8; (f) adult TgAQP1+/+ lens; (g) adult WT lens to show AQP0 expression (positive control for membrane localization). Anti-AQP1 antibody binding (green) to the differentiating and differentiated primary and secondary fiber cells (a-f). The antibody also bound to cornea, ciliary body and retina in embryonic eye. Anti-AQP0 antibody binding (green) to lens fiber cells (g). Selected lens sections were stained with DAPI (Blue) nuclear stain. AE – anterior epithelium; DF – differentiating fiber cells; EC – embryonic cornea; EQ – equatorial epithelial cells; ER – embryonic retina; LC – lens cavity; LE – lens; MF – matured fiber cells; OC – optic cup; PE – posterior epithelial cells; PF – primary embryonic lens fiber cells; S – fiber cell suture. B. Transgene AQP1-EGFP expression in adult mouse (3 months of age) lens. Immunocytochemistry: Optical sections of adult TgAQP1+/+ (a) and WT (b) mouse lenses to show patterns of transgene AQP1 and native AQP0 expression, respectively. In both cases, anti-AQP1 antibody bound (green) to the differentiating and differentiated secondary fiber cells and showed a similar pattern. There was an abrupt decrease in antibody binding at the transition from differentiating fibers to mature fibers, both in the TgAQP1+/+ and WT mice lenses.
3.5. Fiber Cell Membrane Water Permeability, Lens Transparency and Morphology in the TgAQP1 Mice
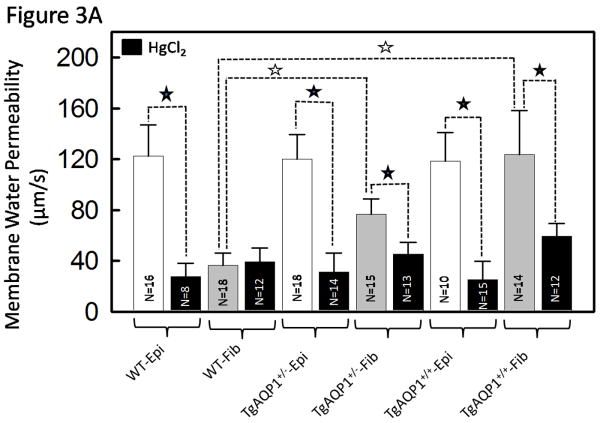
For functional characterization of AQP1 transgene in the lens fiber cells, water permeability of the lens fiber cells of TgAQP1 mouse was studied using membrane vesicles (Varadaraj et al., 1999). Specific membrane water permeability was calculated from the initial rate of volume change when each vesicle was subjected to osmotic shock. Membrane permeability of lens epithelial cells expressing AQP1 in WT and TgAQP1+/+ was ∼120 μm/s and is less than half that of the values obtained in oocyte experiments (Fig. S2B-C). This could be due to the level of functional AQP1 expression, endogenous regulations, difference in cell types (frog egg vs lens epithelial cells) and to some extent the ex vivo experimental conditions. Lens fiber membrane vesicles of WT had an average water permeability value of 36 μm/s due to the AQP0 expression and were insensitive to inhibition by HgCl2. The fiber cells of transgenic AQP1 mice showed ∼3 times more permeability (120 μm/s) compared to that of the WT and responded to inhibition by HgCl2 (Fig. 3A, significance denoted by black stars) as is typical of AQP1. This increase in water permeability was statistically significant (Figure 3A, white stars). The water permeability of the fiber cells of TgAQP1+/+ mouse was comparable to that of the epithelial cells of the TgAQP1+/- and TgAQP1+/+ mice. Compared to the TgAQP1+/+, water permeability of the fiber cells in TgAQP1+/- mouse was less but higher than that in the WT. These results emphasize the specificity as well as the transgenic expression of AQP1 in the fiber cells.
Fig. 3.
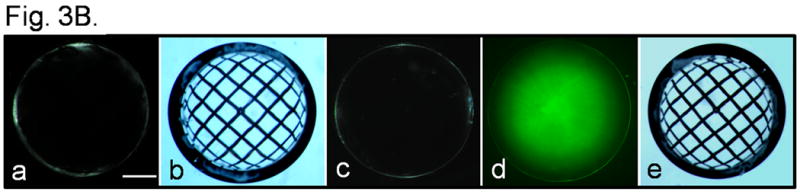
Membrane water permeability and transparency of WT and TgAQP1 mouse lenses. A. Epithelial cell, and fiber cell membrane vesicle water permeability of WT, TgAQP1+/- and TgAQP1+/+ mice lenses. White bars represent epithelial cells and grey bars represent fiber cell vesicles; black bars are epithelial cells or fiber cell membrane vesicles incubated with HgCl2 to see the inhibitory effect on water permeability. Each bar represents the mean ± SD. N, number of cells or membrane vesicles used per experiment. Star represents the degree of significance in comparison, P<0.0001. Epi - Epithelial cells, Fib - Fiber cells. B. Lens transparency of WT and TgAQP1+/+ lenses. (a) and (b), WT; (c-e), TgAQP1+/+. (a), (b), (c) and (e), light microscopic images of lenses; (d), epifluorescent image of lens expressing AQP1-EGFP chimeric protein observed using EGFP fluorescent filter; (b) and (e), lenses focusing copper metal grid. Bar, 275 μm.
After confirming specificity and functionality of transgenic AQP1, we focused on the lenses for positive and/or negative effects of the expression. Lenses of WT (Fig. 3B, a,b) and TgAQP1+/+ (Fig. 3B, c-e) at 2 months of age were compared; there was no significant difference in size, transparency or focusing ability. Lens transparency of WT and TgAQP1+/+ was quantified by contrast ratio analysis of transmitted grid image versus transmitted non-grid image; there was no significant difference in focusing of the grid assuring complete lens transparency (Fig. 3B, compare e to b). TgAQP1+/+ lenses remained transparent throughout the observation period of 18 months.
All of the positive outcomes in the transgenic WT mouse provided motivation and encouragement to induce transgenic AQP1 expression in the lens fiber cells of AQP0 knockout mice by crossing with TgAQP1+/+ mice.
3.6. Generation of Transgenic Mice Expressing AQP1-EGFP Chimeric Transgene in the Lens Fiber Cells of AQP0 Knockout Mice
Pups of AQP0 knockout mice are born with severe lens cataract (Shiels et al., 2001). If this phenotype is the result of the lack of water permeability caused by the deficiency of endogenous AQP0 and if the function of AQP0 is only water conductance, expression of a much more efficient water pore, AQP1, in the fiber cells of AQP0 null mice should logically be able to restore lens transparency in these mice. With this hypothesis as the basis, we crossed TgAQP1+/+ mice with AQP0 knockout (AQP0-/-) mice to develop a transgenic mouse line (TgAQP1+/+/AQP0-/-) constitutively expressing AQP1 in the lens fiber cells. The resulted offspring were intercrossed and analyzed by genotyping using the DNA extracted from tail biopsies as templates for PCR along with a primer mix containing Mip.4, Mip.5, Rv.3 (Shiels et al., 2001), prim1 and prim5 to identify TgAQP1+/+/AQP0-/- mice.
Genomic PCR showed amplification of the expected segments (Fig. 4). From the primer mix, wild-type AQP0 allele (277 bp) was amplified by primers Mip.4 and Mip.5 (Supplemental data, Table S1) indicating the presence of endogenous AQP0 in WT, AQP0+/- and TgAQP1+/-/AQP0+/- (lanes 1, 2 and 4) lenses. AQP0 mutant allele (788 bp, lanes 2-5) of the knockout mice was amplified by primers Mip.4 and Rv.3 and AQP1 transgenic segment (1.9 kb, lanes 4 and 5) by prim1 and prim5. In order to identify homozygous AQP1 transgene expression in AQP0 null mice, positive offspring (TgAQP1+/-/AQP0-/- and TgAQP1+/+/AQP0-/-) were backcrossed with the wild type. The backcrossed offspring which transmitted EGFP fluorescence to the lenses of all pups of a resulting litter was identified as TgAQP1+/+/AQP0-/-. RT-PCR of lens total RNA from TgAQP1+/+/AQP0-/- mouse showed the presence of AQP1-EGFP transcripts. Results are described in detail in the Supplemental Data section (Fig. S4).
Fig. 4.
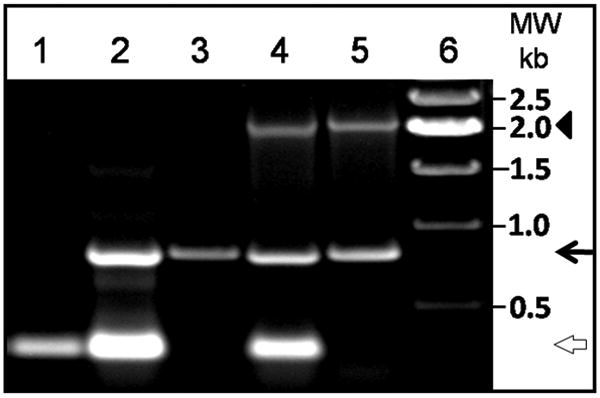
Identification of homozygous AQP1 transgenic AQP0 knockout (TgAQP1+/+/AQP0-/-) mice by genotyping. PCR-based genotyping of heterozygous AQP0 knockout (AQP0+/-), homozygous AQP0 knockout (AQP0-/-), heterozygous AQP1 transgenic AQP0 knockout (TgAQP1+/-/AQP0+/-), and homozygous AQP1 transgenic AQP0 knockout (TgAQP1+/+/AQP0-/-) mice. Samples were loaded as follows: lanes 1 WT; lane 2, AQP0+/-; lane 3, AQP0-/-; lane 4, TgAQP1+/-/AQP0+/-; lane 5, TgAQP1+/+/AQP0-/-; lane 6, molecular weight marker. White open arrow, 277 bp segment; black arrow, 788 bp segment; arrowhead, 1.9 kb segment.
3.7. AQP1-EGFP Protein Expression in the Lenses of TgAQP1+/+/AQP0-/- Mice
The expression of AQP1-EGFP transgene in the developing and adult lenses of TgAQP1+/+/AQP0-/- mice was verified by immunocytochemical studies using anti-AQP1 antibodies which revealed membrane localization of the protein in the postnatal (Fig. 5A, b) and adult (Fig. 5A, c) lenses. TgAQP1+/+/AQP0-/- mouse lenses showed considerable improvement in the fiber cell quality (Fig. 5A, c) compared to the AQP0-/- lenses (Fig. 5A, d); however, irregularly shaped cells as well as vacuolated cells were still present. More degenerating fiber cells were present in the inner cortex and nuclear regions of the lens. In contrast, WT lens sections contained healthy, compactly packed and uniform fiber cells (Fig. 5A, e). Despite the improvement in the overall fiber cell quality compared to the AQP0-/- lenses, TgAQP1+/+/AQP0-/- lenses failed to achieve the ordered compact arrangement of fiber cells along the polar axis and the uniformity in size.
Fig. 5.
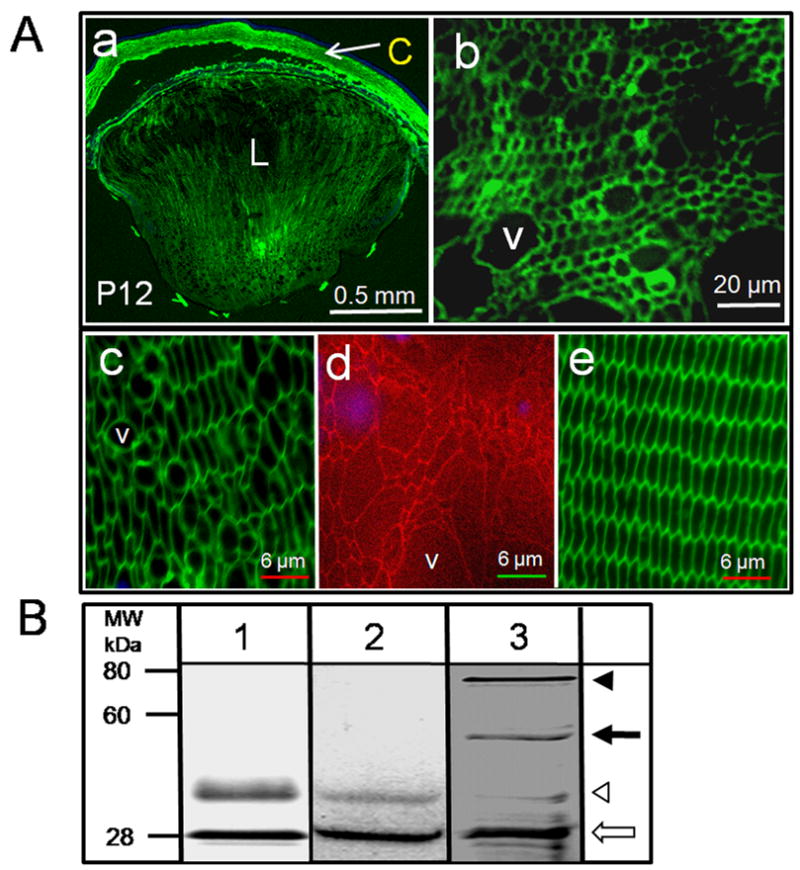
Expression of aquaporin protein. A. Immunostaining for transgene AQP1-EGFP expression in the developing lens of TgAQP1+/+AQP0-/- transgenic mouse using anti-AQP1 antibody; a, sagittal section (polar plane) through the eye at P12 (anterior pole facing up); b-e, cross section at equatorial plane through the lens; b, at P12; c, TgAQP1+/+AQP0-/- adult lens cortex; d, AQP0-/- adult lens cortex stained with WGA-Texas Red-X to show fiber cell structure; e, WT adult lens cortex immunostained for AQP0 using anti-AQP0 as a positive control for membrane localization. Nuclei stained with DAPI (Blue). Antibody binding in Green. L - Lens, C – Cornea, V – Vacuole. B. Immunoblotting. Total membrane proteins from kidney (lane 1; WT - positive control), and lens from WT (lane 2) and TgAQP1+/+AQP0-/- (lane 3) mouse were analyzed by immunoblotting using polyclonal anti-AQP1 antibodies. Positions to which the molecular mass standards migrated are indicated on the left. White arrow - AQP1 protein (28 kDa); white arrowhead - AQP1 glycosylated form (∼40 kDa); Black arrow - AQP1-EGFP chimeric protein (∼55 kDa); Black arrowhead - AQP1-EGFP glycosylated form (∼70 kDa).
Qualitative western blotting (Fig. 5B) of mouse total membrane protein fractions isolated separately from adult lenses showed specific anti-AQP1 binding to a 28-kDa band (white arrow) corresponding to the nonglycosylated AQP1 and a ∼40 kDa band (white arrowhead) corresponding to the glycosylated form in the WT (lane 2) and TgAQP1+/+/AQP0-/- (lane 3) mice, reflecting the expression of endogenous AQP1 in the lens epithelial cells; similar bands were observed for the control membrane preparations from kidney (lane 1). In addition, TgAQP1+/+/AQP0-/- lens membrane fractions revealed two more immunopositive bands of ∼55 and ∼70 kDa corresponding to the nonglycosylated and glycosylated forms of AQP1-EGFP chimeric protein, respectively (Fig. 5B lanes 3, black arrow and black arrowhead) confirming the expression of the transgene in the lens fiber cells. Comparable immunoreactive bands were observed by Hoque et al. (2000) for cells expressing AQP1-GFP.
The level of expression of the nonglycosylated and glycosylated forms of endogenous AQP1 (indicated by white arrow and white arrowheads, respectively in lane 3), is not identical to that of AQP1-EGFP chimeric protein (indicated by black arrow and black arrowheads, respectively in lane 3) in the fiber cells of TgAQP1+/+/AQP0-/- mouse lens. This may be due to the difference in protein expression in a non-native cell type (fiber cells as opposed to the epithelial cells) and/or the chimeric nature of the protein. Different tissues express different levels of glycosylated AQP1. For example, kidney has more glycosylated AQP1 (lane 1) than the endogenous AQP1 from the lens epithelial cells (lane 2). It has been reported that both glycosylated and non-glycosylated forms function as water channels with equivalent efficiencies (van Hoek et al., 1995). In addition, transgenic expression of AQP1 in the WT (TgAQP1+/+) mouse did not affect lens transparency and morphology or reduce fiber cell membrane water permeability indicating that the level of glycosylation did not interfere with the normal functioning of the transgene AQP1.
3.8. Body Weight, Eye Weight, Lens Weight, Lens Water Content, Eye and Lens Mid-Diameter in Different Mice Phenotypes
Growth and several parameters of eyes and lenses of the different mice phenotypes with reference to aquaporin expression in the lens were determined and compared to assess the pros and cons of the transgenic expression; results as graphs are included in the Supplemental Data section (Fig. S5). Body weight (a) did not show any significant difference among WT, AQP0-/-, TgAQP1+/+ or TgAQP1+/+/AQP0-/- mice. AQP0-/- showed significant decrease in eye wet weight (b), lens wet weight (c), lens dry weight (d), water content in the lens (e), eye mid-diameter (f) and lens mid-diameter (g) compared to those of the WT or TgAQP1+/+. When TgAQP1+/+/AQP0-/- was compared with AQP0-/-, there was significant increase in all of the tested parameters except body weight and between WT and TgAQP1+/+, there was no significant difference. In TgAQP1+/+/AQP0-/- and WT, eye wet weight, lens dry weight, water content in the lens and eye mid-diameter were very similar; lens wet weight and lens mid-diameter of TgAQP1+/+/AQP0-/- were significantly less than in the WT. In contrast to AQP0-/- mouse, lenses of TgAQP1+/+/AQP0-/- mouse regained most of the water content and dry weight, with values closer to those of WT and TgAQP1+/+ mice. Transgenic expression of AQP1 in the fiber cells of AQP0-/- mouse significantly improved the lens quality compared to the AQP0-/- lens.
3.9. Lens Transparency and Morphology
Lenses from prenatal to adults of WT, AQP0+/-, AQP0-/-, TgAQP1+/-, TgAQP1+/+, TgAQP1+/-/AQP0+/- and TgAQP1+/+/AQP0-/- mice were observed under light microscope; selected images are given as Figure 6. Wild type lenses were transparent (Fig. 6A, a,b) from P30 to 18-months of age (maximum age at which observations were made). AQP0+/- mouse showed gradual increase in lens opacity (Fig. 6A, c) as the lens development progressed (Fig. 6B b,g); homozygous AQP0 knockout mice showed higher level of lens opacity and cataract in a gene dose-dependent manner (d (homozygous), compare with c (heterozygous)). Both TgAQP1+/- (e) and TgAQP1+/+ (f) lenses were transparent from P30 to 18-months of age and showed EGFP fluorescence in a dose-dependent manner (i, j). TgAQP1+/-/AQP0+/- (g) and TgAQP1+/+/AQP0-/- (h) lenses exhibited improvement in lens transparency compared to AQP0 knockout lenses (Fig. 6A, compare (g) with (c) and (h) with (d)) and also showed the intensity of EGFP fluorescence in a dose dependent manner (k, l); with progression in age, the level of lens transparency gradually decreased as seen for AQP0 knockout mouse lenses (Fig. 6B; compare (b) with (g), (c) with (h), (d) with (i) and (e) with (j)). Lens transparency of the TgAQP1+/-/AQP0+/- and TgAQP1+/+/AQP0-/- lenses improved significantly relative to the AQP0+/- and AQP0-/- lenses; however, it did not measure up to that in the WT (Fig. 6B, a,f), as evidenced by the quantification analysis (Fig. 6C).
Fig. 6.
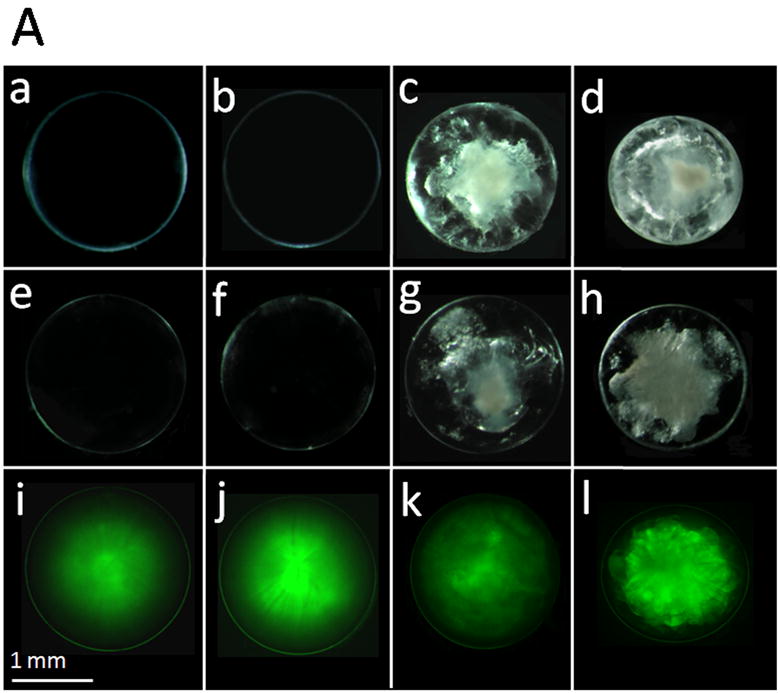
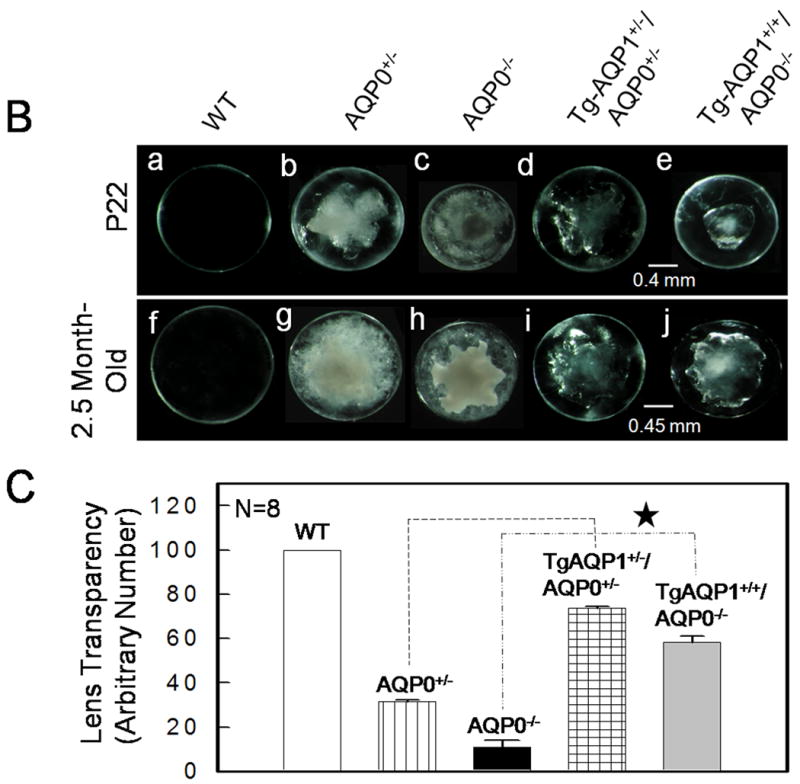
Lens transparency in relation to aquaporin expression. A. Lens transparency, cataract and AQP1-EGFP expression in 2-month old mice. (a,b), WT; (c), AQP0+/-; (d), AQP0-/-; (e,i), TgAQP1+/-; (f,j), TgAQP1+/+; (g,k), TgAQP1+/-/AQP0+/-; (h,l) TgAQP1+/+/AQP0-/-. (a-h), light microscopic images; (i-l), epifluorescent images of lenses expressing AQP1-EGFP chimeric protein. B. Relation between the age, and presence or absence of AQP0 and AQP1 in lens transparency and severity of cataract. Light microscopic images of lenses: (a,f), WT; (b,g), AQP0+/-; (c,h), AQP0-/-; (d,i) TgAQP1+/-/AQP0+/-; (e,j), TgAQP1+/+/AQP0-/-. C. Quantification of lens transparency at age P22 in the presence or absence of AQP0 and AQP1 in the lens fiber cells. N, number of lenses used to collect data. Star represents the degree of significance in comparison, P<0.0001.
3.10. Fiber Cell Membrane Water Permeability in the TgAQP1+/+/AQP0-/- Mouse
Water permeability of fiber cell membrane vesicles was determined in presence of Ca2+ as described by Varadaraj et al. (1999, 2007) and was 36 ± 10 μm/s for WT due to AQP0; it could not be inhibited by HgCl2 (39 ± 11 μm/s; Fig. 7). AQP0+/- and AQP0-/- lenses exhibited water permeability values of 20 ± 5 and 8 ± 3 μm/s, respectively; this is ∼2 and 5 fold less respectively, compared to the WT. TgAQP1+/-/AQP0+/- and TgAQP1+/+/AQP0-/- mouse lenses had fiber cell membrane water permeability values of 61 ± 13 and 95 ± 11 μm/s, respectively and these amount to 3 and 12 fold increase respectively, compared to AQP0+/- and AQP0-/- mouse lenses. Membrane water permeability of TgAQP1+/-/AQP0+/- and TgAQP1+/+/AQP0-/- increased significantly by 1.7 and 2.6 fold respectively relative to the WT lenses; this increase reflects the expression of transgenic AQP1 in the fiber cells; as further evidence, the water permeability was blocked significantly by HgCl2 (Fig. 7).
Fig. 7.
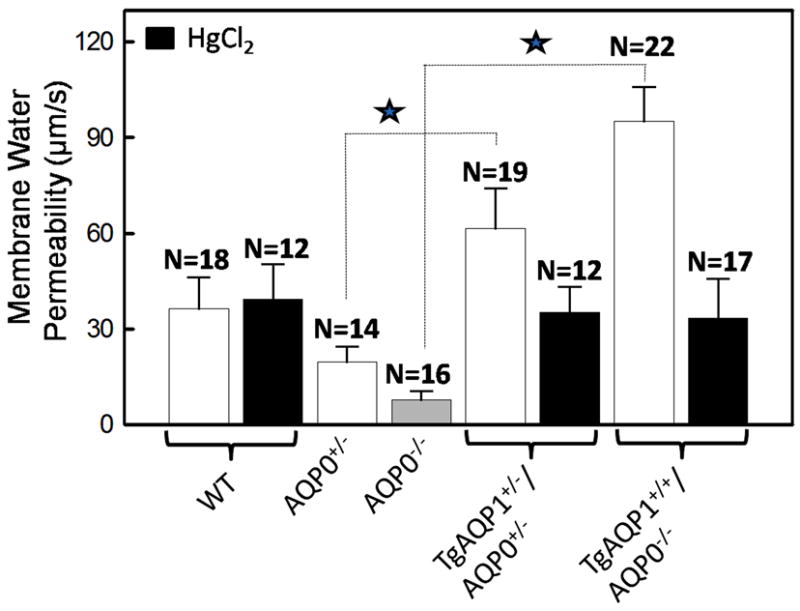
Lens membrane water permeability in the presence or absence of aquaporins. Fiber cell membrane water permeability of 4-month old WT, AQP0+/-, AQP0-/-, TgAQP1+/-/AQP0+/- and TgAQP1+/+/AQP0-/- mice. In some experiments, the fiber cell membrane vesicles were incubated with HgCl2 to inhibit AQP1. Each bar represents mean ± SD. N, number of membrane vesicles used per experiment. Star represents the degree of significance in comparison, P<0.0001.
AQP1 expression in the fiber cells of AQP0-/- mice increased the water permeability and improved the overall lens morphology and transparency but lens cataract development was not eliminated and the fiber cell uniformity and ordered architecture were not restored (Fig. 5A b,c).
3.11. Cell-to-cell adhesion capability of AQP0 and AQP1
Prompted by the disorganization of the fiber cells in TgAQP1+/+/AQP0-/- mice, we sought to explore the cell-to-cell adhesion capabilities of AQP0 and AQP1, with and without the EGFP tag. AQP0 (Fig. 8A a-d) and AQP0-EGFP (e-h) significantly promoted cell-to-cell aggregation while AQP1 (i-l) and AQP1-EGFP (m-p) did not. Cell aggregation capability of AQP0 and AQP0-EGFP was comparable to that of the positive control cells expressing E-cadherin (Fig. 8B) whereas AQP1 and AQP1-EGFP were comparable to the negative control (empty vector) with no aggregates. EGFP tag in both AQP0 and AQP1 did not alter the respective cell-to-cell adhesion property.
Fig. 8.
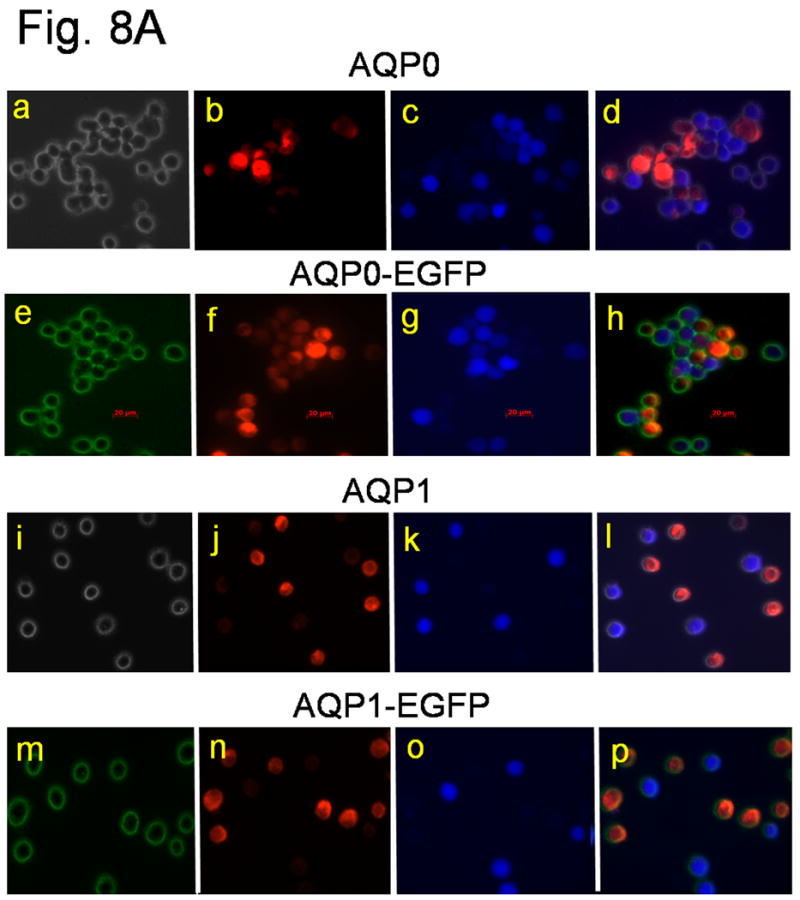
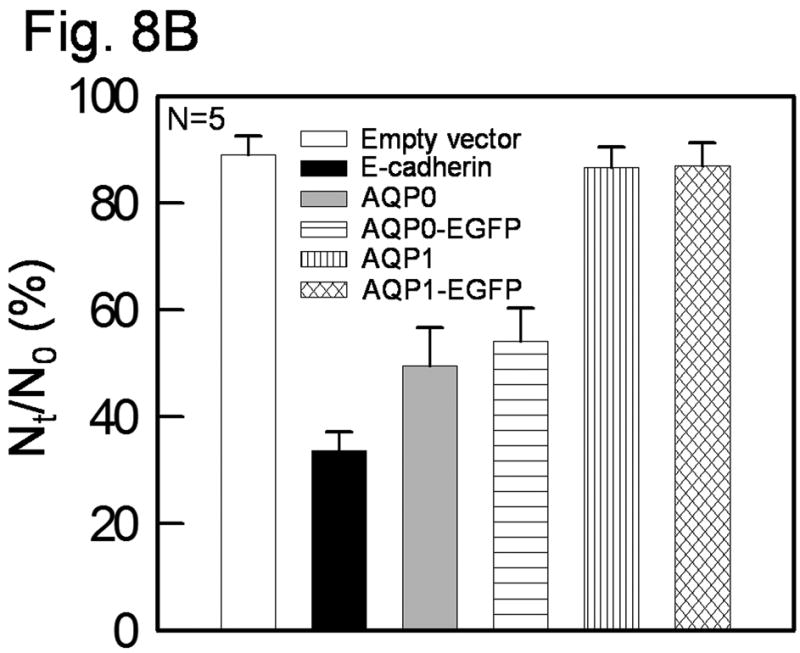
Cell aggregation assay using rotary gyratory shaker. (A) Bi-color fluorescence aggregation assay using L-cells expressing mouse AQP0 (a-d), AQP0-EGFP (e-h), human AQP1 (i-l) and AQP1-EGFP (m-p). (a) and (i), phase contrast images; others epifluorescent images. (e) and (m), fluorescence due to EGFP tag. (b, f, j, n), cells loaded with CellTracker Red; (c, g, k, o) cells loaded with CellTracker Blue. d, h, l and p merged images of (a,b,c), (e,f,g), (i,j,k) and (m,n,o), respectively. (B) Histogram showing cell aggregation induced by adhesion deficient L-cells expressing mouse AQP0 with or without EGFP tag and human AQP1 with or without EGFP tag and E-cadherin (positive control), after 90 min. of incubation on a gyratory shaker.
4. Discussion
The role of AQP0 in the ocular lens had long been under speculation and several functions such as cell-to-cell coupling, ionic conductance, water permeability and cell-to-cell adhesion were proposed (reviewed in Chepelinsky, 2009). Using Xenopus oocyte in vitro system several researchers have demonstrated the water channel function of AQP0 (Chandy et al., 1997; Kushmerick et al., 1995; Mulders et al., 1995; Varadaraj et al., 2005, 2008; Yang and Verkman, 1997; Zampighi et al., 1995). Based on single channel studies it has been estimated that AQP1 is ∼40 times more efficient in conducting water than AQP0 (Chandy et al., 1997). However, the lens switches protein expression from AQP1 in the equatorial epithelial cells to the less efficient water channel, AQP0, in the differentiating secondary fiber cells. The reason could be, while AQP0 has water permeability as a shared function, it may have function/s of unique nature that cannot be fulfilled by AQP1 and hence its presence in the fiber cells is essential for endowing and maintaining lens transparency and homeostasis. Indirect evidences indicate that AQP0 may play a structural role as a cell-to-cell adhesion protein. However, there are no in vivo experiments to demonstrate that besides being a water channel, AQP0 performs an additional function that is critical for lens transparency. Our study was undertaken to fill this void. If AQP0, which is capable of cell-to-cell adhesion in vitro, performs water permeability as the sole function to contribute towards lens transparency, then, transgenic expression of AQP1 - which lacks the cell-to-cell adhesion capability - in the fiber cells should be able to restore lens transparency in the cataractous lenses of AQP0 null mouse. If not, the inference would be AQP0 has a unique function, probably cell-to-cell adhesion, in the fiber cells.
Various transgenic mouse lines generated by independent research groups for characterizing specific proteins in the lens, exhibited lens defects; however, only a few developed cataracts. Transgenic expression of wild type myocilin (Zillig et al., 2005), desmin (Dunia et al., 1990) or low levels of Na+/myo-inositol co-transporter (Cammarata et al., 1999) in the lens did not lead to cataract. Lenses of several transgenic mouse models showed abnormalities (Cammarata et al., 1999; Capetanaki et al., 1989; Chung et al., 2007; Duncan et al., 2004; Dunia et al., 1990; Perez-Castro et al., 1993; West-Mays et al., 2002). However, in the current study, there were no morphological or anatomical abnormalities or cataract in the lenses of AQP1 transgenic wild type (TgAQP1+/+) mice. The lenses were transparent and normal in size, shape and structure as in the wild type assuring the functional competence as well as the innocuous nature of transgene AQP1 in the fiber cells despite a 3-fold increase in membrane water permeability. The results also suggest that reducing the water permeability might not be the purpose behind the switching of protein expression from AQP1 to AQP0 during fiber cell differentiation since the increased water permeability did not affect lens transparency or morphology.
Total fiber cell membrane vesicle water permeability of transgenic knockout mouse (TgAQP1+/+/AQP0-/-) lenses was 2.6 times more than that of wild type fiber cells expressing AQP0. This increase is moderate since we have used αA-crystallin promoter to avoid very high levels of overexpression which otherwise could cause detrimental effects. Even though the estimated single channel water permeability of AQP1 in the oocyte is 40 times higher than that of AQP0 (Chandy et al., 1997) ourselves and others including Chandy et al., did not see a corresponding increase when total oocyte membrane water permeabilities of the two channels obtained through experimentation were compared; the increase was 4-6 times compared to AQP0 when expressed in oocytes and 3-fold when the total membrane water permeabilities between lens epithelial and fiber cells were compared. The discrepancy could be due to the presence of only a few functional AQP1 channels in the plasma membrane. We have selected a particular transgenic founder to keep the fiber cell membrane water permeability equal to or slightly higher than that of the wild type expressing AQP0. The physiological impact of this reasonable increase could be an improvement in lens water/ion flow possibly adding to the fiber cell quality and viability of the TgAQP1+/+/AQP0-/- mouse lenses. There were remarkable positive differences such as increase in lens size, transparency and membrane water permeability compared to AQP0-/- mouse lenses. Degeneration of the fiber cells was less progressive with age in TgAQP1+/+/AQP0-/- lenses and there was an overall improvement in the fiber cell quality at the outer cortex in comparison to AQP0-/- lens. However, lenses of TgAQP1+/+/AQP0-/- mouse did not match those of the normal wild type in size, morphology or transparency suggesting that AQP1, despite being a more efficient water channel, could not replace AQP0 functionally. Therefore, it is reasonable to conclude that in addition to the analogous function of being a water channel, AQP0 serves at least one unique function that cannot be replaced or compensated by AQP1 transgene.
Our previous data show that AQP0 and AQP1 are expressed spatially and temporally in the lens (Varadaraj et al., 2007). AQP0 began to express in the primary fiber cells at E11.25 as they differentiate and its synthesis continued through the adult stage in the secondary fiber cells. The onset of AQP1 expression occurred in the anterior epithelial cells only at E17.5 even though epithelial cells are formed much earlier than the fiber cells during embryonic development. AQP0 is expressed as the primary fiber cells begin to form; switching of protein expression from AQP1 in the epithelial cells to AQP0 in the differentiating secondary fiber cells indicates that this genetically programmed transition could be a prelude to assure the presence of AQP0 in all of the fiber cells presumably to carry out a specific function. Fiber cell deformation in TgAQP1+/+/AQP0-/- lens (Fig. 5A b,c) indicates lack of cell-to-cell adhesion. In the TgAQP1+/+/AQP0-/- lens, the required membrane water permeability is more than compensated by the transgene AQP1. Therefore, loss of membrane water permeability may not be the only reason for lens cataract development in the AQP0-/- mice. Several investigators have proposed that AQP0 may function as a cell-to-cell adhesion protein (Costello et al., 1985; Fotiadis et al. 2000; Gonen et al., 2005; Harries et al., 2004; Michea et al., 1994, 1995; Zampighi et al., 1982, 1992). Recently, we have demonstrated the cell-to-cell adhesion property of AQP0 in vitro, using adhesion-deficient L-cells (Kumari and Varadaraj, 2009). AQP0 and AQP0-EGFP are capable of cell-to-cell adhesion while AQP1 and AQP1-EGFP are not (Figure 8). Our results indicate that the additional and unique function of AQP0 could be providing cell-to-cell adhesion for the fiber cells. AQP0 may start with an adhesion function in the primary fiber cells, continue the same for the secondary fiber cells as they differentiate in order to create and maintain an ordered and compact cellular architecture conducive for minimizing light scattering; as the lens grows bigger in size, it contributes to the water permeability of the fiber cells when there is a higher demand for water permeability.
Using the TgAQP1+/+/AQP0-/- mouse model we have shown in vivo that the transgenic expression of AQP1 in the fiber cells could restore only partial lens transparency, despite compensating the required water permeability. Therefore, it appears that presence of AQP0, which possess the capability of cell-to-cell adhesion (Kumari and Varadaraj, 2009) in addition to water conductance, is necessary for regaining complete lens transparency. As yet, there is no in vivo study to demonstrate that AQP0 participates in cell-to-cell adhesion apart from being a water channel. Besides, there is no specific inhibitor to block the water channel function of AQP0 in order to test for other role(s). We are currently investigating the possible structural role of AQP0 as a cell-to-cell adhesion protein in vivo by developing appropriate animal models.
Supplementary Material
Acknowledgments
We thank Dr. Peter Agre, Department of Cell Biology, Duke University Medical Center, Durham, NC for providing human AQP1 cDNA, Dr. Ana B. Chepelinsky, NIH for αA-crystallin promoter and Dr. Alan Shiels, Washington University, St. Louis, MO for AQP0 knockout mouse. This work was supported by National Institute of Health (Grant numbers: R01: EY020506 (K.V.); and Alcon Research Ltd., (39733, K.V.) and R01: EY06391 (R.T.M).
Abbreviations
- AQP0
Aquaporin 0
- AQP1
Aquaporin 1
- TgAQP1
wild type mouse expressing transgene AQP1 in the lens fiber cells
- TgAQP1+/+/AQP0-/-
AQP0 knockout mouse expressing transgene AQP1 in the lens fiber cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski RK, Shiels A, Kuszak JR. Lens structure in MIP-deficient mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:714–730. doi: 10.1002/ar.a.10080. [DOI] [PubMed] [Google Scholar]
- Ball LE, Little M, Nowak MW, Garland DL, Crouch RK, Schey KL. Water permeability of C-terminally truncated aquaporin 0 AQP0 1-243 observed in the aging human lens. Invest Ophthalmol Vis Sci. 2003;44:4820–4828. doi: 10.1167/iovs.02-1317. [DOI] [PubMed] [Google Scholar]
- Cammarata PR, Zhou C, Chen G, Singh I, Reeves RE, Kuszak JR, Robinson ML. Transgenic animal model of osmotic cataract. Part 1: over-expression of bovine Na+/myo-inositol cotransporter in lens fibers Invest. Ophthalmol Vis Sci. 1999;40:1727–1737. [PubMed] [Google Scholar]
- Capetanaki Y, Smith S, Heath JP. Overexpression of the vimentin gene in transgenic mice inhibits normal lens cell differentiation. J Cell Biol. 1989;109:1653–1664. doi: 10.1083/jcb.109.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membr Biol. 1997;159:29–39. doi: 10.1007/s002329900266. [DOI] [PubMed] [Google Scholar]
- Chepelinsky AB. Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts. Handb Exp Pharmacol. 2009;190:265–297. doi: 10.1007/978-3-540-79885-9_14. [DOI] [PubMed] [Google Scholar]
- Chung J, Berthoud VM, Novak L, Zoltoski R, Heilbrunn B, Minogue PJ, Liu X, Ebihara L, Kuszak J, Beyer EC. Transgenic overexpression of connexin 50 induces cataracts. Exp Eye Res. 2007;84:513–528. doi: 10.1016/j.exer.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MJ, McIntosh TJ, Robertson JD. Membrane specializations in mammalian lens fiber cells: distribution of square arrays. Curr Eye Res. 1985;4:1183–1201. doi: 10.3109/02713688509003364. [DOI] [PubMed] [Google Scholar]
- Detmers FJM, de Groot BL, Müller EM, Hinton A, Konings IBM, Sze M, Flitsch SL, Grubmüller H, Deen PMT. Quaternary ammonium compounds as water channel blockers: specificity, potency, and site of action. J Biol Chem. 2006;281:14207–14214. doi: 10.1074/jbc.M513072200. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Xie L, David LL, Robinson ML, Taube JR, Cui W, Reneker LW. Ectopic Pax6 expression disturbs lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2004;45:3589–3598. doi: 10.1167/iovs.04-0151. [DOI] [PubMed] [Google Scholar]
- Dunia I, Pieper F, Manenti S, van de Kemp A, Devilliers G, Benedetti EL, Bloemendal H. Plasma membrane-cytoskeleton damage in eye lenses of transgenic mice expressing desmin. Eur J Cell Biol. 1990;53:59–74. [PubMed] [Google Scholar]
- Fotiadis D, Hasler L, Muller DJ, Stahlberg H, Kistler J, Engel A. Surface tongue-and-groove contours on lens MIP facilitate cell-to-cell adherence. J Mol Biol. 2000;300:779–789. doi: 10.1006/jmbi.2000.3920. [DOI] [PubMed] [Google Scholar]
- Francis P, Berry V, Bhattacharya S, Moore A. Congenital progressive polymorphic cataract caused by a mutation in the major intrinsic protein of the lens, MIP AQP0. Br J Ophthalmol. 2000;84:1376–1379. doi: 10.1136/bjo.84.12.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerometta R, Zamudio AC, Escobar DP, Candia OA. Volume change of the ocular lens during accommodation. Am J Physiol Cell Physiol. 2007;293:C797–804. doi: 10.1152/ajpcell.00094.2007. [DOI] [PubMed] [Google Scholar]
- Geyer DD, Spence MA, Johannes M, Flodman P, Clancy KP, Berry R, Sparkes RS, Jonsen MD, Isenberg SJ, Bateman JB. Novel single-base deletional mutation in major intrinsic protein MIP in autosomal dominant cataract. Am J Ophthalmol. 2006;141:761–763. doi: 10.1016/j.ajo.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen T, Cheng YF, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Zhai H, Li D, Zhao L, Li C, Huang S, Ma X. A novel mutation in major intrinsic protein of the lens gene (MIP) underlies autosomal dominant cataract in a Chinese family. Mol Vis. 2007;13:1651–1656. [PubMed] [Google Scholar]
- Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc Natl Acad Sci USA. 2004;101:14045–14050. doi: 10.1073/pnas.0405274101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1994. [Google Scholar]
- Hoque AT, Liu X, Kagami H, Swaim WD, Wellner RB, O'Connell BC, Ambudkar IS, Baum BJ. Construction and function of a recombinant adenovirus encoding a human aquaporin 1-green fluorescent protein fusion product. Cancer Gene Ther. 2000;7:476–485. doi: 10.1038/sj.cgt.7700146. [DOI] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, Valiunas V, Ramanan SV, Christensen EA, Beyer EC, Brink PR. Functional expression and biophysical properties of polymorphic variants of the human gap junction protein connexin37. Biochem Biophys Res Commun. 2000;274:216–224. doi: 10.1006/bbrc.2000.3054. [DOI] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, Valiunas V, Brink PR. Site-directed mutations in the transmembrane domain M3 of human connexin 37 alter channel conductance and gating. Biochem Biophys Res Commun. 2001;280:440–447. doi: 10.1006/bbrc.2000.4121. [DOI] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K. Intact AQP0 performs cell-to-cell adhesion. Biochem Biophys Res Commun. 2009;390:1034–1039. doi: 10.1016/j.bbrc.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick C, Rice SJ, Baldo GJ, Haspel HC, Mathias RT. Ion, water and neutral solute transport in Xenopus oocytes expressing frog lens MIP. Exp Eye Res. 1995;61:351–362. doi: 10.1016/s0014-4835(05)80129-0. [DOI] [PubMed] [Google Scholar]
- Lin H, Hejtmancik JF, Qi Y. A substitution of arginine to lysine at the COOH-terminus of MIP caused a different binocular phenotype in a congenital cataract family. Mol Vis. 2007;13:1822–1827. [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P. The lens circulation. J Membr Biol. 2007;216:1–16. doi: 10.1007/s00232-007-9019-y. [DOI] [PubMed] [Google Scholar]
- Michea LF, de la Fuente M, Lagos N. Lens major intrinsic protein (MIP) promotes adhesion when reconstituted into large unilamellar liposomes. Biochemistry. 1994;33:7663–7669. doi: 10.1021/bi00190a021. [DOI] [PubMed] [Google Scholar]
- Michea LF, Andrinolo D, Ceppi H, Lagos N. Biochemical evidence for adhesion-promoting role of major intrinsic protein isolated from both normal and cataractous human lenses. Exp Eye Res. 1995;61:293–301. doi: 10.1016/s0014-4835(05)80124-1. [DOI] [PubMed] [Google Scholar]
- Mulders SM, Preston GM, Deen PMT, Guggino WB, Vanos CH, Agre P. Water channel properties of major intrinsic protein of lens. J Biol Chem. 1995;270:9010–9016. doi: 10.1074/jbc.270.15.9010. [DOI] [PubMed] [Google Scholar]
- Németh-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275:6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- Okamura T, Miyoshi I, Takahashi K, Mototani Y, Ishigaki S, Kon Y, Kasai N. Bilateral congenital cataracts result from a gain-of-function mutation in the gene for aquaporin-0 in mice. Genomics. 2003;81:361–368. doi: 10.1016/s0888-7543(03)00029-6. [DOI] [PubMed] [Google Scholar]
- Perez-Castro AV, Tran VT, Nguyen-Huu MC. Defective lens fiber differentiation and pancreatic tumorigenesis caused by ectopic expression of the cellular retinoic acid-binding protein I. Development. 1993;119:363–375. doi: 10.1242/dev.119.2.363. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Verkman AS. Accelerated cataract formation and reduced lens epithelial water permeability in aquaporin-1-deficient mice. Invest Ophthalmol Vis Sci. 2006;47:3960–3967. doi: 10.1167/iovs.06-0229. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bassnett S. Mutations in the founder of the MIP gene family underlie cataract development in the mouse. Nat Genet. 1996;12:212–215. doi: 10.1038/ng0296-212. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bassnett S, Varadaraj K, Mathias RT, Al-Ghoul K, Kuszak J, Donoviel D, Lilleberg S, Friedrich G, Zambrowicz B. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol Genomics. 2001;7:179–186. doi: 10.1152/physiolgenomics.00078.2001. [DOI] [PubMed] [Google Scholar]
- Simirskii VN, Lee RS, Wawrousek EF, Duncan MK. Inbred FVB/N mice are mutant at the cp49/Bfsp2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci. 2006;47:4931–4934. doi: 10.1167/iovs.06-0423. [DOI] [PubMed] [Google Scholar]
- van Hoek AN, Wiener MC, Verbavatz JM, Brown D, Lipniunas PH, Townsend RR, Verkman AS. Purification and structure-function analysis of native, PNGase F-treated, and endo-beta-galactosidase-treated CHIP28 water channels. Biochemistry. 1995;34:2212–2219. doi: 10.1021/bi00007a015. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Skinner DM. Denaturants or cosolvents improve the specificity of PCR amplification of a G+C-rich DNA using genetically-engineered DNA-polymerases. Gene. 1994;140:1–5. doi: 10.1016/0378-1119(94)90723-4. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Skinner DM. Actin-encoding cDNAs and gene expression during the intermolt cycle of the Bermuda land crab Gecarcinus lateralis. Gene. 1996;171:177–184. doi: 10.1016/0378-1119(96)00018-2. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Skinner DM. Molecular characterization of four members of the alpha-tubulin gene family of the Bermuda land crab Gecarcinus lateralis. J Exp Zool. 1997;278:63–77. [PubMed] [Google Scholar]
- Varadaraj K, Kushmerick C, Baldo GJ, Bassnett S, Shiels A, Mathias RT. The role of MIP in lens fiber cell membrane transport. J Membr Biol. 1999;170:191–203. doi: 10.1007/s002329900549. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest Ophthalmol Vis Sci. 2005;46:1393–1402. doi: 10.1167/iovs.04-1217. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Mathias RT. Functional expression of aquaporins in embryonic, postnatal, and adult mouse lenses. Dev Dyn. 2007;236:1319–1328. doi: 10.1002/dvdy.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Patil R, Wax MB, Mathias RT. Functional characterization of a human aquaporin 0 mutation that leads to a congenital dominant lens cataract. Exp Eye Res. 2008;87:9–21. doi: 10.1016/j.exer.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Coyle BM, Piatigorsky J, Papagiotas S, Libb D. Ectopic expression of AP-2α transcription factor in the lens disrupts fiber cell differentiation. Dev Biol. 2002;245:13–27. doi: 10.1006/dbio.2002.0624. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- Yang Y, Stopka T, Golestaneh N, Wang Y, Wu K, Li A, Chauhan BK, Gao CY, Cveklová K, Duncan MK, Pestell RG, Chepelinsky AB, Skoultchi AI, Cvekl A. Regulation of αA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. EMBO J. 2006;25:2107–2118. doi: 10.1038/sj.emboj.7601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi G, Simon SA, Robertson JD, McIntosh TJ, Costello MJ. On the structural organization of isolated bovine lens fiber junctions. J Cell Biol. 1982;93:175–189. doi: 10.1083/jcb.93.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Simon SA, Hall JE. The specialized junctions of the lens. Int Rev Cytol. 1992;136:185–225. doi: 10.1016/s0074-7696(08)62053-7. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Kreman M, Boorer KJ, Loo DD, Bezanilla F, Chandy G, Hall JE, Wright EM. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J Membr Biol. 1995;148:65–78. doi: 10.1007/BF00234157. [DOI] [PubMed] [Google Scholar]
- Zillig M, Wurm A, Grehn FJ, Russell P, Tamm ER. Over expression and properties of wild-type and Tyr437His mutated myocilin in the eyes of transgenic mice. Invest Ophthalmol Vis Sci. 2005;46:223–234. doi: 10.1167/iovs.04-0988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.