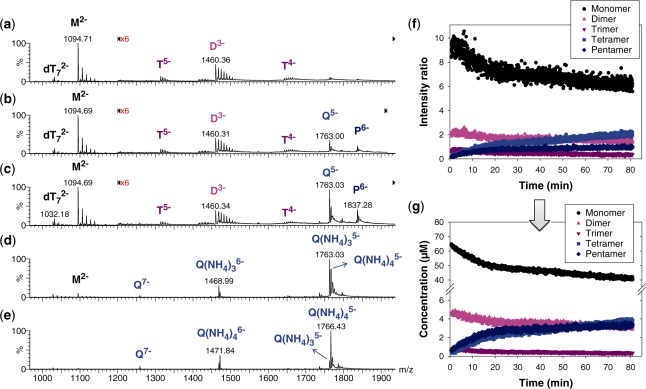
Figure 3.
Kinetics of G-quadruplex formation by 80 µM dTG5T in 150 mM NH4OAc and 10% methanol. (a–e) Electrospray mass spectra recorded 2 min (a), 10 min (b), 60 min (c), 25 days (d) and 164 days (e) after ammonium acetate addition. M stands for monomer, D for dimer, T for trimer, Q for tetramer, P for pentamer. Note the magnification from 1200 to 2000 m/z in spectra (a–c). (f) Intensity ratio measured between a characteristic peak of each stoichiometry (M2− for monomer, D3− for the dimer, T5− for the trimer, Q5− for the tetramer and P6− for the pentamer) and the reference peak 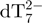 , as a function of time elapsed after ammonium addition. (g) Time evolution of the concentration of each stoichiometry, obtained after correcting for the relative response factor of each characteristic peak (note the break on the y-axis).
, as a function of time elapsed after ammonium addition. (g) Time evolution of the concentration of each stoichiometry, obtained after correcting for the relative response factor of each characteristic peak (note the break on the y-axis).