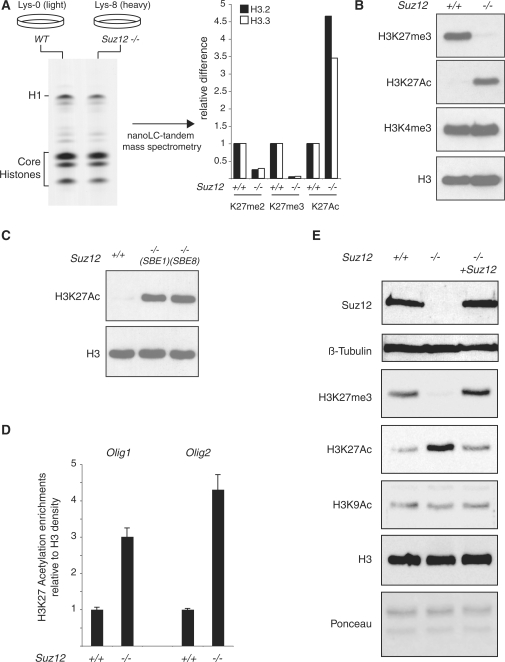
Figure 1.
Loss of Suz12 induces H3K27 hyperacetylation. (A) (left panel) Coomassie-blue staining of SILAC-labeled histones purified from light-isotope-labeled (Lys-0) WT ES cells and heavy-isotope-labeled (Lys-8) Suz12 KO ES cells. Nanolc-tandem mass spectrometry quantification of the K27 methylation and acetylation levels of H3.2 and H3.3 in WT and Suz12 KO ES cells (right panel). (B) Western blot analyses of histones purified from WT and Suz12 KO ES cells using the indicated antibodies. H3 is presented as loading control. (C) Western blot analyses of histones purified from WT and two independent Suz12 KO ES cell lines using the indicated antibodies. H3 is presented as loading control. (D) ChIP analysis of the Olig1 and Olig2 promoter in WT and Suz12 KO ES cells using an H3K27Ac-specific antibody. H3K27Ac signal is normalized to histone density using an H3-specific antibody. (E) Western blot analyses of total protein extracts obtained from WT and Suz12 KO ES cell before and after CRE expression using the indicated antibodies. β-tubulin, H3 and Ponceau staining are presented as loading controls.