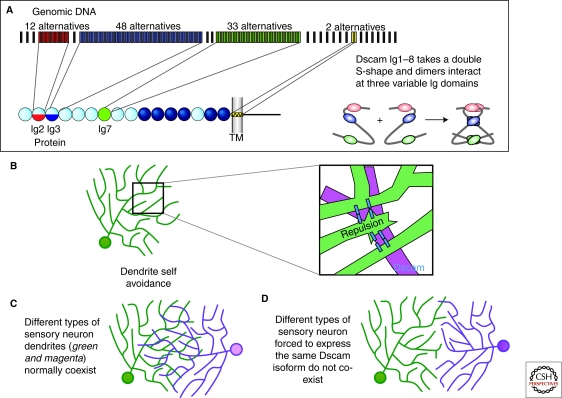
Figure 2.
Dscam regulates dendrite self-avoidance. (A) The Dscam locus can generate up to 152,064 distinct protein isoforms by alternative splicing, including 19,008 distinct extracellular domains (Schmucker et al. 2000; Yu et al. 2009). Exons 4 and 6 code for half Ig domains (Ig2 and Ig3 respectively) and exon 9 codes for all of Ig7. Extensive binding assays have shown isoform-specific homophilic binding (Wojtowicz et al. 2004; Wojtowicz et al. 2007). The structure of the homophilic binding region (including Ig1-Ig8) indicates that the protein folds into an S shaped molecule, and that this folding allows interactions between the three variable Ig domains. Adapted from (Sawaya et al. 2008). (B) Studies in several systems (see text) indicate that Dscam is critical for self-avoidance of dendrites and axons. Studies are consistent with a model in which sister dendrites or axons that encounter one another during development are recognized by virtue of their shared isoform repertoire. This recognition leads to repulsive signaling, the molecular basis of which is not yet understood. (C) Single Dscam isoforms are sufficient for self-recognition and avoidance but Dscam diversity is required between cells so that they can share territories (coexistence). Left: Two different neurons with coexisting arbors. When those cells are forced to express the same arbitrary isoform at high levels their arbors no longer cross each other and are thus unable to coexist (see text for details).