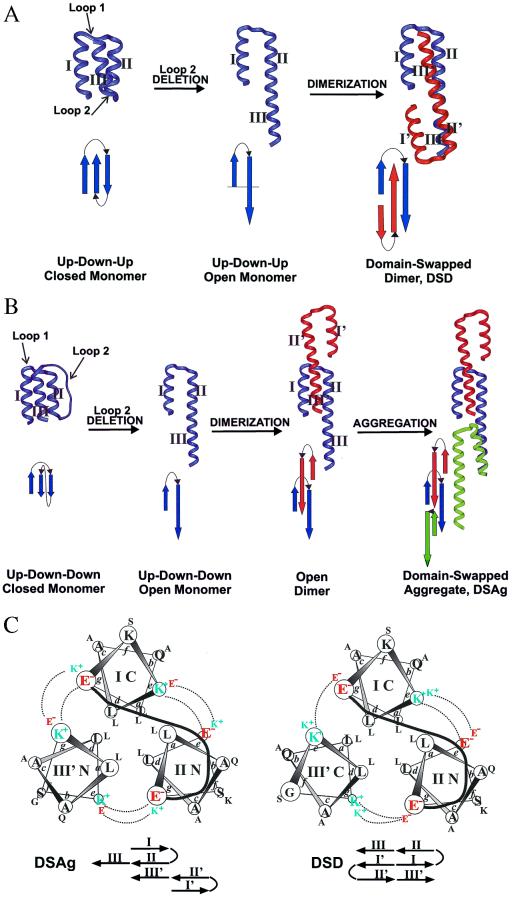
Figure 1.
Design of up-down-up and up-down-down three-helix bundles, and their domain-swapped counterparts. (A) Design of a DSD, beginning with Mon1 (up-down-up topology). (B) Design of a domain-swapped open aggregate, starting with Mon2 (up-down-down topology). (C) Helical wheel diagrams and amino acid sequence of DSAg and DSD. Each helical wheel diagram illustrates a single functional unit. The topologies of the functional units of DSAg and DSD differ only with respect to the orientation of helix III′ (antiparallel to helix I in DSAg and parallel in DSD). The positions of the Glu and Lys residues at the helix interfaces have been arranged to differentially stabilize the two different topologies. Notice that the leucine-containing hydrophobic cores are identical. In the amino acid sequences of DSD and DSAg, the leucine core (a and d heptad positions) are the same. Only the charged e and g positions are redistributed to reorient the molecules.