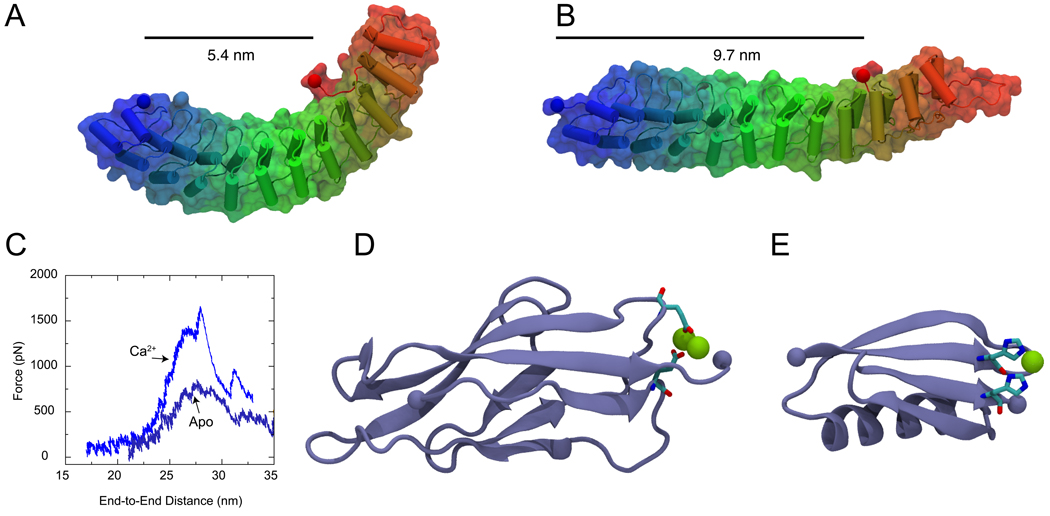
Figure 3.
Tertiary structure elasticity of Ankyrin-R and divalent-controlled secondary structure elasticity of cadherin and GB1 proteins. (A) Snapshot of an Ankyrin-R domain in its crystallographic conformation (Michaely et al., 2002). The elongated and curved shape results from parallel stacking of 12 ankyrin repeats. The protein is shown in cartoon and surface representation. (B) Stretched conformation of Ankyrin-R obtained through SMD simulations (Sotomayor et al., 2005). The protein reversibly changes its shape without modifying its secondary structure elements. The end-to-end distance measured from terminal Cα atoms (taking into account the spectrin binding domain) is shown before and after stretching. (C) The unfolding force as a function of distance is shown for C-cadherin simulations performed in the presence (Ca2+) and absence (Apo) of calcium. A significant reduction in the force required to unfold cadherin repeats is observed in the absence of calcium. (D) Snapshot of the first C-cadherin repeat structure (Boggon et al., 2002) shown in cartoon representation. Calcium ions (shown in green) link β-strands A and F through highly conserved and charged amino-acids (shown in licorice representation). (E) Structure of GB1 protein illustrating the position of an engineered histidine-metal chelation site at position G8-55, based on (Cao et al., 2008). (F) Topology of cadherin repeats. A link between β-strands A and F is formed in the presence of Ca2+. (G) Similarly, a link between GB1 β-strands A and D is formed upon addition of Ni2+. The links enhance the mechanical stability of these proteins.