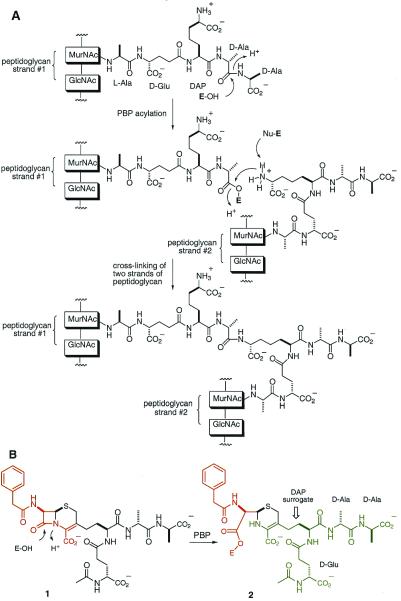
Figure 1.
(A) A representative transpeptidation reaction for Gram-negative bacteria and some Gram-positives catalyzed by certain penicillin-binding proteins (PBPs), beginning with acylation of the active-site serine of the enzyme by the peptide of one strand of peptidoglycan (E denotes the enzyme). Reaction of the second strand of the peptidoglycan with the ester of the acyl-enzyme intermediate results in the cross-linked cell wall. (B) The backbone of cephalosporin 1 (in red) mimics the terminal acyl-d-Ala-d-Ala portion of the peptide branch of the first strand of the peptidoglycan. The C-7 acyl moiety, the phenylacetyl group, is that seen in penicillin G. Cephalosporin 1 acylates the active site serine of the transpeptidase, as would the peptide from the first strand of peptidoglycan, concomitant with the departure of the terminal d-Ala. The β-lactam nitrogen and its adjacent carbon and carboxylate collectively serve as a surrogate for the departing d-Ala, and the same atoms constitute a portion of the incoming DAP surrogate in strand 2 in complex 2. The acyl-enzyme species 2 depicts the first enzyme-bound “peptidoglycan” strand (shown in red) poised to receive the amine of DAP from the second strand of peptidoglycan (shown in green).