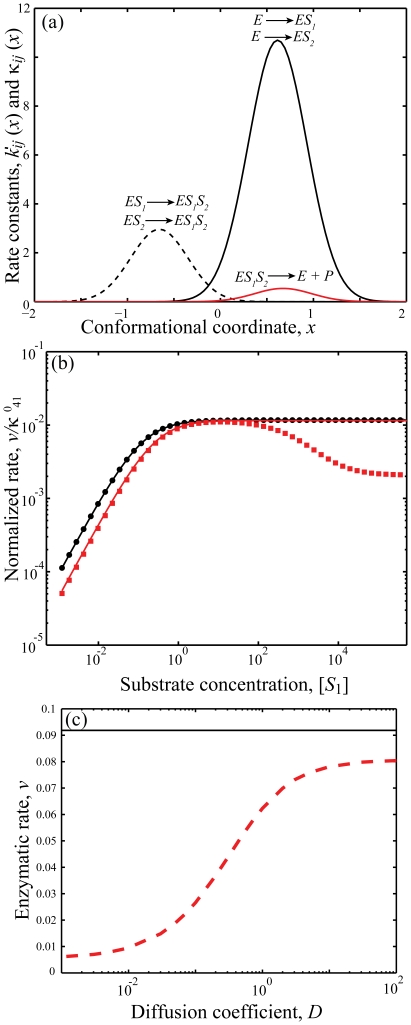
Figure 2. Numerical results for the random order bisubstrate reaction.
(a) Rate constants κij (x) and kij (x) as function of the conformational coordinate x, with parameters given in Tables 1 and 2 calculated from Eq. (34) and 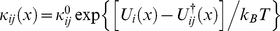 . (b) Normalized rate
. (b) Normalized rate 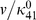 as a function of concentration [S1] at a fixed concentration of [S2] = 10. When catalytic reaction is fast with
as a function of concentration [S1] at a fixed concentration of [S2] = 10. When catalytic reaction is fast with 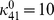 , slow diffusion in ES conformation leads to non-monotonic dependence —substrate inhibition effect (red squares) and a deviation from the macroscopic rate law (red solid line) computed from mass action kinetics. For slow catalysis (
, slow diffusion in ES conformation leads to non-monotonic dependence —substrate inhibition effect (red squares) and a deviation from the macroscopic rate law (red solid line) computed from mass action kinetics. For slow catalysis (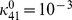 , quasi-equilibrium limit) normalized rate
, quasi-equilibrium limit) normalized rate 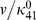 has the same dependence on S1 concentration (black circles) as the macroscopic kinetic law (black solid line) calculated from equation (B.4). We use DES1 = 10−2 and DE = DES2 = DES1ES2 = 102 and the rest of parameters as in (a). c) Enzymatic rate as a function of conformational diffusion. We took all the diffusion coefficients to be the same DE = DES1 = DES2 = DES1S2 = D. For fast catalysis
has the same dependence on S1 concentration (black circles) as the macroscopic kinetic law (black solid line) calculated from equation (B.4). We use DES1 = 10−2 and DE = DES2 = DES1ES2 = 102 and the rest of parameters as in (a). c) Enzymatic rate as a function of conformational diffusion. We took all the diffusion coefficients to be the same DE = DES1 = DES2 = DES1S2 = D. For fast catalysis 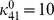 the enzymatic rate decreases with decreased diffusion (red dashed line). In the quasi-equilibrium limit, when the catalysis is slow
the enzymatic rate decreases with decreased diffusion (red dashed line). In the quasi-equilibrium limit, when the catalysis is slow 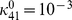 the enzymatic rate does not depend on the diffusion coefficient (black solid line). Notably the same trend continues with further decrease in diffusion coefficients, D. We use [S1] = [S2] = 1 and the remaining parameters as in (a, b).
the enzymatic rate does not depend on the diffusion coefficient (black solid line). Notably the same trend continues with further decrease in diffusion coefficients, D. We use [S1] = [S2] = 1 and the remaining parameters as in (a, b).