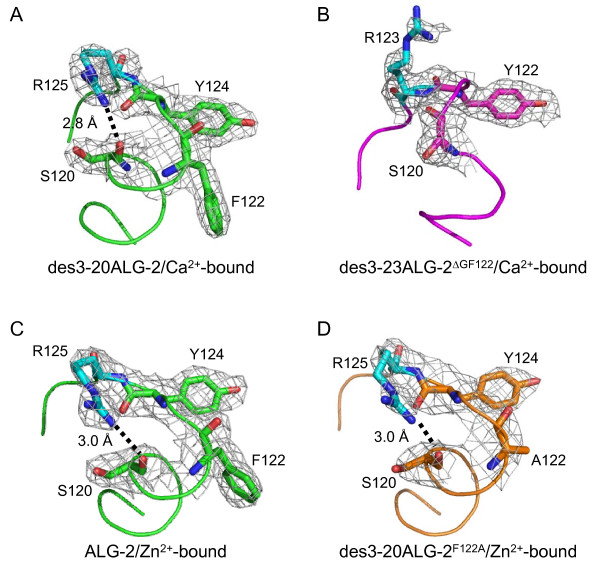
Figure 2.
Differences in the side chain configurations of R125 and R123. Close-up views of a segment from L115 to D128 (D126 in des3-23ALG-2ΔGF122) in each ALG-2 structure are shown in ribbon representations and in stick models superimposed with electron densities of residues that are involved in the Ca2+/EF3-driven arginine switch mechanism. The structures of (A, C) wild-type ALG-2, (B) des3-23ALG-2ΔGF122 and (D) des3-20ALG-2F122A are colored green, magenta and orange, respectively. R125, a critical residue for the switch mechanism and interaction with Alix, and its corresponding residue in des3-23ALG-2ΔGF122 (R123) are colored cyan. The hydrogen bond between the guanidino nitrogen atom of R125 and carbonyl oxgen atom of S120 is shown by a dotted line and the distance is indicated in (A), (C) and (D).