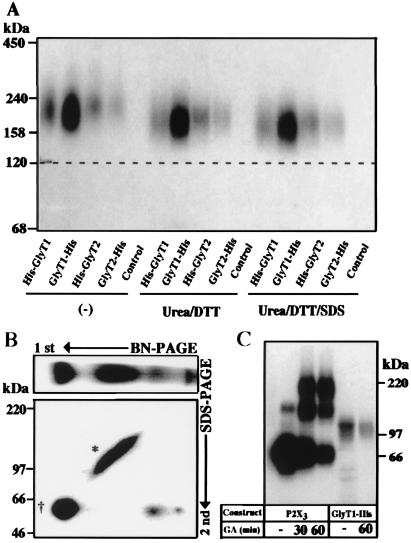
Figure 4.
Surface GlyTs migrate as monomeric proteins. (A) Effect of reduction, urea, and SDS on the mobility of surface-labeled GlyT proteins. After surface labeling with [15I]sulfo-SHPP and affinity purification, the 125I-labeled GlyTs were incubated at room temperature either alone (Left) or with 100 mM DTT and 8 M urea in the absence (Center) or presence (Right) of 0.1% (wt/vol) SDS, respectively, before analysis by 4–10% BN/PAGE. Note that the mobility of the labeled proteins did not change significantly under the different conditions. (B) An aliquot of a detergent extract prepared from an GlyT1-His-injected oocyte after metabolic labeling with [35S]methionine as shown in Fig. 2 was subjected to two-dimensional PAGE. After BN/PAGE on a 4–10% acrylamide gradient gel, the separated proteins were resolved in the second dimension by 8% SDS/PAGE. * and †, the complex-glycosylated and core-glycosylated forms, respectively. (C) Surface-labeled oocytes expressing either the P2X3 receptor or GlyT1-His were incubated with 10 mM glutaraldehyde (GA) for the indicated periods before extraction and affinity purification. The purified proteins were then analyzed on a 4–10% SDS/polyacrylamide gradient gel. Note the formation of P2X3 dimers and trimers, whereas no adducts were seen with GlyT1-His.