Scheme 2.
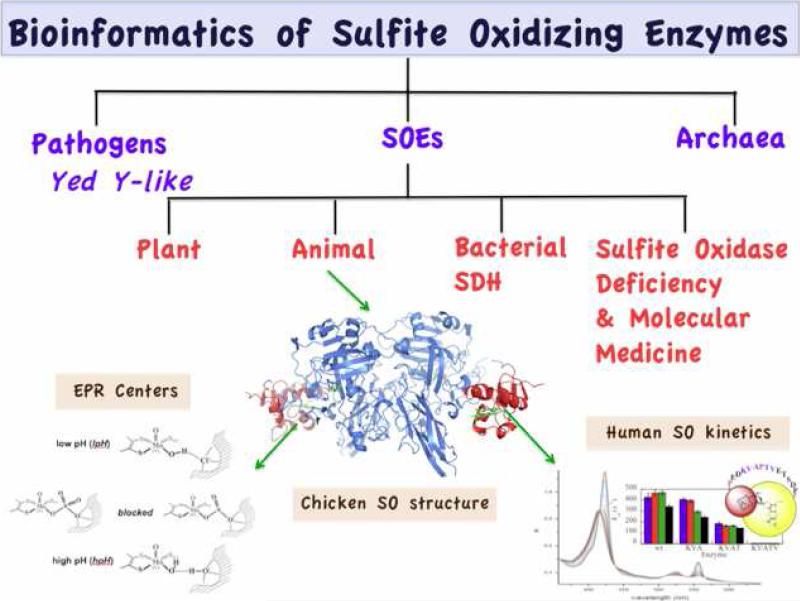
Overview of the current state of research on sulfite oxidizing enzymes (SOEs). From bioinformatic analyses, the diverse sulfite oxidase family of proteins that contains the same molybdenum cofactor center (1) can be classified into at least three groups (63, 85). This review has focused on the SOEs. Animal sulfite oxidases possess two redox active domains and present fundamental biophysical problems relating to intramolecular electron transfer (IET), the relationship of IET rates to steady-state kinetics, the overall conformation of the protein, and the molecular dynamics of the motion of the two domains relative to one another. The effects of extensive mutations of the tether connecting the heme and molybdenum domains of human SO have been discussed here. However, as yet there are no X-ray structural results of the intact protein for any of these variants. The detailed structures of the molybdenum centers of these variants have been determined from analysis of high resolution pulsed EPR spectra of their Mo(V) states as a function of pH, anions in the media, and mutations of nearby amino acid residues. A long term hope is that these studies will contribute to the continuing development of molecular medicine to treat sulfite oxidase deficiency (13, 16).