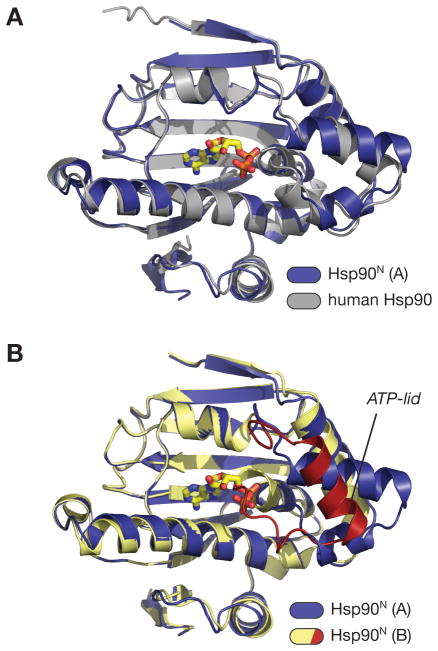
Figure 2. Hsp90N comparisons.
(A) Superposition of the A (ADP-bound) monomer of Hsp90N (blue; bound ADP in yellow) with the structure of the human Hsp90 N-terminal domain bound to geldanamycin (PDB ID 1YET) (gray; bound geldanamycin not shown). The overall r.m.s.d. of the two domains is 0.79 Å. (B) Superposition of the A (ADP-bound) and B (apo) monomers of Hsp90N. Monomer A is colored blue, and monomer B is colored yellow with the ATP-lid red. The conformation of the ATP-lid in monomer B precludes nucleotide binding, as residues 118–121 would directly overlap with the α- and β-phosphates of ADP.