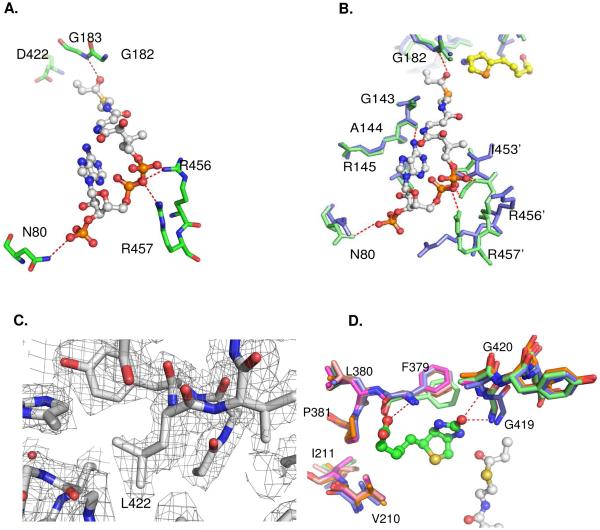
Fig. 3.
(A) Interactions of the CoA phosphate groups with residues N80, R456 and R457 via hydrogen bonds in wild type PccB. (B) Enlarged view, sructural overlap between the wild-type (green) and D422N mutant (blue), showing the conformational changes near the 55 – 70 and 450 – 460 loops. (C) The Fo-Fc simulated annealing electron density map near residue 422 for D422L, contoured at 2.5 sigma. (D) Structural overlap of the biotin binding pockets of all the PccB mutant shows that there is no significant conformational change in the biotin binding pocket. In the enlarged panels (B), residue 422 is out of sight, but is located above the viewpoint.