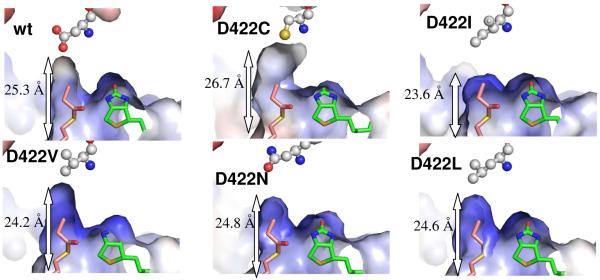
Fig. 4.
A comparison of acyl-CoA binding pocket between wild-type and PccB mutants. The molecular surface is colored from red to blue according to electronegative to electropositive surface potential. The labeled length measures the distance from the entrance (the N80 side chain) to the bottom (residue 422 side chain) of the acyl-CoA binding pocket. As a molecular rule, propionyl-CoA and biotin were docked into the apo mutant structures based on the co-crystal structure of the wild type substrate-biotin complex.