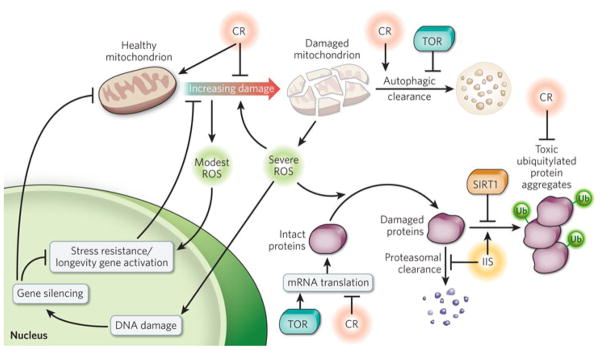
Figure 3. Conserved pathways that regulate organismal and brain ageing.
Shown are mechanisms that involve mitochondrial function, oxidative stress, autophagy, protein homeostasis, TOR signalling, insulin/IGF-1 signalling (IIS), caloric restriction (CR) and sirtuins. Modest concentrations of ROS generated by mitochondria during normal metabolism may induce stress-resistance pathways that scavenge ROS and repair damage. However, progressive mitochondrial damage may lead to pathological concentrations of ROS production, which, in turn, may contribute to further mitochondrial damage. Damaged mitochondria can be cleared by autophagy, which is promoted by CR and inhibited by TOR signalling. CR improves overall mitochondrial function, in part, by promoting mitochondrial biogenesis and reducing ROS production (100). ROS can damage other crucial macromolecules, such as DNA and proteins. Unrepaired DNA damage may give rise to epigenetic changes and gene silencing and may exacerbate mitochondrial impairment by reducing the expression of nuclear-encoded mitochondrial genes. ROS can also modify proteins, leading to protein unfolding and aggregation. Modified proteins can be removed by a number of degradative pathways, including the ubiquitin proteasome pathway. Inadequate clearance may lead to the accumulation of toxic protein aggregates. The dynamics of protein clearance and aggregate formation may be modulated by the IIS pathway and by SIRT1 and CR. The accumulation of damaged and toxic proteins may also be modified through the regulation of messenger RNA translation by TOR signalling and CR.