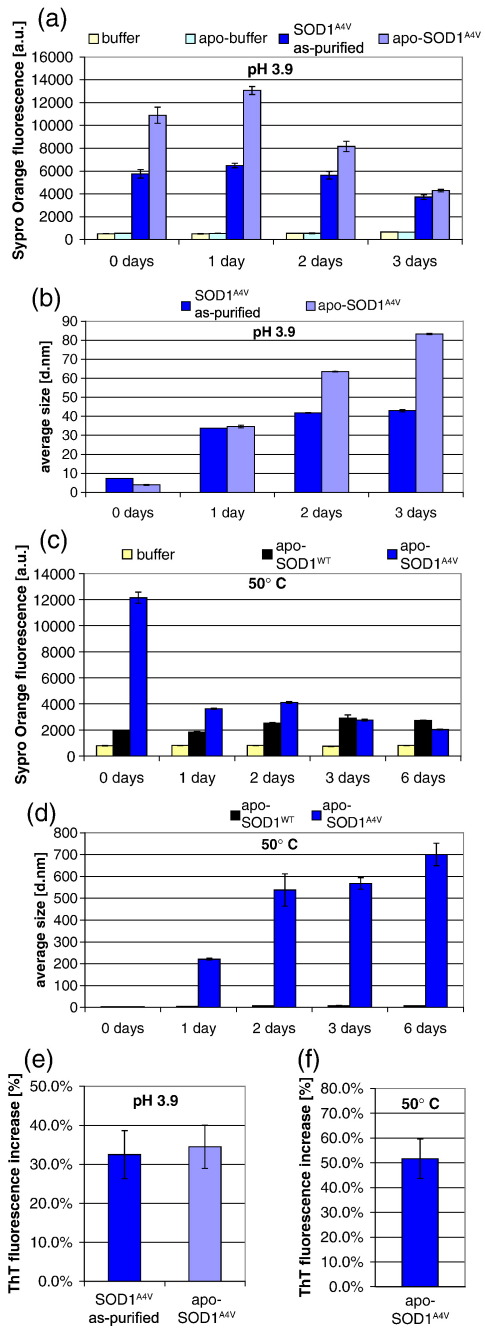
Fig. 5.
Aggregation of as-purified SOD1 mutants follows exposure of hydrophobic surfaces. (a) Time course of measurements of exposed hydrophobicity monitored by Sypro Orange fluorescence and (b) aggregation of SOD1A4V at acidic pH monitored by DLS. Note that demetallation first provoked a decrease in the size of particles measured, indicating that metal loss provoked monomerization of the protein. (c) Time course of measurements of exposed hydrophobicity and (d) aggregation of apo-SOD1A4V exposed to 50 °C. (e, f) aggregation of SOD1A4V monitored by ThT after 3 days of incubation at acidic pH (e) or 50 °C (f). Data are means and s.d. values of replicate experiments (n = 3).