Abstract
The effects of stress, including their putative contribution to pathological psychiatric conditions, are crucially governed by the age at which the stress takes place. However, the cellular and molecular foundations for the impact of stress on neuronal function, and their change with age, are unknown. For example, it is not known whether ‘psychological’ stress signals are perceived by similar neuronal populations at different ages, and whether they activate similar or age-specific signaling pathways that might then mediate the spectrum of stress-evoked neuronal changes. We employed restraint and restraint/noise stress to address these issues in juvenile (postnatal day 18, [P18]) and adult rats, and used phosphorylation of the transcription factor CREB (pCREB) and induction of c-fos as markers of hippocampal neuronal responses. Stress-activated neuronal populations were identified both anatomically and biochemically, and selective blockers of the stress-activated hippocampal peptide, corticotropin-releasing hormone (CRH) were used to probe the role of this molecule in stress-induced hippocampal cell activation. Stress evoked strikingly different neuronal response patterns in immature vs adult hippocampus. Expression of pCREB appeared within minutes in hippocampal CA3 pyramidal cells of P18 rats, followed by delayed induction of Fos protein in the same cell population. In contrast, basal pCREB levels were high in adult hippocampus and were not altered at 10–120 min by stress. Whereas Fos induction was elicited by stress in the adult, it was essentially confined to area CA1, with little induction in CA3. At both age groups, central pretreatment with either a nonselective blocker of CRH receptors (α-helical CRH [9–41]) or the CRF1-selective antagonist, NBI 30775, abolished stress-evoked neuronal activation. In conclusion, hippocampal neuronal responses to psychological stress are generally more rapid and robust in juvenile rats, compared to fully mature adults, and at both ages, CRH plays a key role in this process. Enhanced hippocampal response to stress during development, and particularly the activation of the transcription factor CREB, may contribute to the enduring effects of stress during this period on hippocampal function.
Keywords: CRH, CRH receptor, CRF, CRF1, CREB, pCREB, Fos, transcription factor, hippocampus
Introduction
It is well established that the effects of stress on the central nervous system vary as a function of the age at which stress is imposed (reviewed in McEwen,1 Sanchez et al.,2 Welberg and Seckl,3 Avishai-Eilner et al.,4 Levine,5 Miller and O’Callaghan6 and Fenoglio et al.7). For example, prenatal stress has been shown to ‘re-program’ or ‘imprint’ both neuroendocrine and behavioral responses to subsequent stress throughout the lifetime (see Avishai-Eilner et al.,4 Fenoglio et al.,7 Wadhwa et al.,8 Welberg et al.9 and Weinstock10 for recent reviews). Neuroendocrine activation in response to stress also varies as a function of age. For example, early in postnatal life, hormonal responses to some stressors may be lower than during adulthood.5,11,12 In addition, the ‘adolescent’ period has been characterized by enhanced sensitivity to the effects of stress, with potential relevance to the pathophysiology of addictive behaviors and/or schizophrenia.13,14 Finally, during aging, stress-evoked glucocorticoids may provoke more profound loss or dysfunction of neurons.15–19
Focusing on the effects of stress on the hippocampal formation, age-dependent consequences of ‘psychological’ stress may be governed by the maturity of stress-responsive hippocampal circuits20–22 as well as by other undefined age-specific vulnerabilities. In addition, the age-related differential effects of stress on hippocampal neurons may be attributable to the fact that stressful signals reach and influence different neuronal populations in immature and adult brain. In other words, the specific neuronal populations that are activated by the stress signal and/or the type of signaling cascades that are elicited by the stress within these neurons may contribute to the influence of this signal on the function of the hippocampal network. In accord with this notion, the ability of stress signals to evoke transcription-factor phosphorylation in, for example, the hypothalamus, has been found to be age-dependent,23,24 and the sensitivity of stress-regulated genes to stress ‘signals’ is also a function of developmental age.25–27 Therefore, it is reasonable to expect that differential involvement of hippocampal neurons by stress, or the differential activation of selective immediate-early genes or transcription factors by the stress signal, will result in distinct impact on hippocampal integrity and function.
Several potential mediators of ‘psychological’ stress-evoked modulation of hippocampal neuronal function have been demonstrated. Glucocorticoid hormones bind to their cognate receptors, primarily within hippocampal CA1, and elicit a large number of cellular responses.28 These include changes of synaptic function and plasticity,29,30 dendritic remodeling 31,32 and, in large amounts, neuronal injury.33–35 More recently, activation of mineralocorticoid receptors has been found to mediate certain stress effects on the hippocampus.36 A second candidate for mediating the effects of psychological stress on the hippocampus is CRH, because this peptide is involved in both systemic and brain-specific actions of stress.37–40 This 41 amino-acid peptide was originally isolated from the hypothalamus, where it is rapidly released from CRH-expressing neuronal populations in the para-ventricular nucleus upon physical and physiological stress.41,42 In both mature and immature organisms of several mammalian species, the role of hypothalamic CRH in stress-evoked elevation of plasma glucocorticoids, via activation of CRH receptors within the pituitary gland, has been well established.2,25,41,43
Contribution of CRH to the effects of stress on neuronal function within the brain has also been delineated, and demonstrates age-dependent properties. For example, central administration of CRH activates neurons in the amygdala, contributing to anxiety-like behaviors44,45 as well as to memory consolidation.46 The peptide modulates neuronal activity in amygdala in a complex manner47 and, in larger amounts, can lead to hyperexcitability, a kindling effect, and seizures.48,49 A role for CRH as an effector mediating the complex effects of stress on the hippocampal formation has been emerging. The peptide is synthesized in subsets of hippocampal neurons23,50 and is released by stress into the synaptic space to activate CRH receptors.51 Interestingly, the number and distribution of CRH-expressing inter-neurons within hippocampus is strongly age-dependent, and significantly higher in juvenile compared to mature rat.23 Finally, recent support for the critical contribution of hippocampal CRH to the action of stress on hippocampal neurons has been provided by studying mice deficient in components of the CRH-signaling cascade (e.g. Coste et al.,52 Bale et al.53 and Refojo et al.54).
Taken together, the facts mentioned above suggest that the age-related differential impact of stress on the hippocampal formation may involve age-specific mediators, activation of different neuronal population, initiation of age-specific molecular cascades within target neurons, or a combination of these processes. These possibilities were evaluated in the current studies. We found that stress-induced neuronal responses required CRH-receptor binding in both immature and adult rats. However, neuronal populations and intracellular mediators were differentially evoked in mature and juvenile hippocampus, likely contributing to the established age-specific nature and longevity of the effects of stress on the hippocampal network.
Materials and methods
Animals
Animals were studied at two ages: Immature rats were studied on postnatal day 18 (P18), when the density of CRH-expressing neurons in hippocampal pyramidal cell layers is maximal.23 These were compared to mature (3-month old) adults. Sprague–Dawley-derived rats were born and maintained in a quiet, uncrowded, temperature controlled NIH approved facility on a 12 h light/dark cycle, with access to lab chow and water ad libitum. Litters were culled to 12 pups if necessary, and adults were housed individually. All experiments were in compliance with National Institutes of Health guidelines and were approved by Institutional Animal Care and Use Committee.
Experimental design: stress and surgical procedures
Psychological restraint stress was imposed on P18 rats: Rats (n = 12) were placed in a restrainer (fashioned from a 50 ml plastic cylindrical tube) for 30 min. To evaluate whether the patterns of neuronal activation induced by psychological stress were model specific, an additional paradigm (n = 25) was used, which consisted of crowding (five animals in a cage measuring 25 × 15 × 10 cm3) and jostling by placing the cage on a Laboratory Rotator (model 1314; Laboratory-Line Instruments, Inc., Melrose Park, IL, USA). The rotator also provided a noisy environment. The neuronal populations activated by these two stress maneuvers were virtually indistinguishable, as was the temporal course measured using both the phosphorylated transcription factor CREB and the immediate early gene c-fos. Therefore, the data from these two groups were combined in the Results section. Adult rats (n = 18) were subjected to a 30 min restraint stress only, using Plexiglas restrainers. For both groups, controls consisted of litter-mates that were killed under relatively ‘stress-free’ conditions.23 Briefly, rats were left undisturbed for 24 h prior to experiments, and were then deeply anesthetized with sodium pentobarbital (100 mg/kg intra-peritoneally) within 45 s of entry into the animal facility. Trunk blood was collected in all groups at the time of killing for analysis of plasma corticosterone levels by radioimmunoassay (ICN, Irvine, CA, USA) as previously described.25,55 The stress paradigms increased plasma corticosterone levels at both age groups to a similar degree (see Figures 4 and 5).
Figure 4.
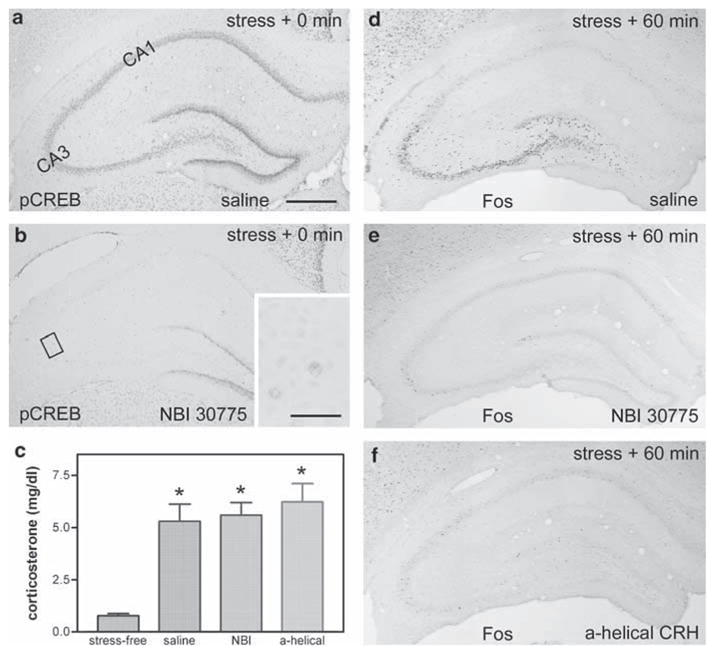
CRF1 receptor antagonists block stress-induced pCREB and Fos expressions in immature hippocampus. (a,b) The CRF1 antagonist NBI 30775 blocks stress-induced pCREB expression in the hippocampus. In a rat pretreated with saline, pCREB is highly expressed in CA3 (and CA1) immediately after the termination of a 30-min stress (a). This stress-induced pCREB expression is blocked (fully in CA3, partially in CA1) by infusion of NBI 30775 (b). (c) The peripheral, hormonal response to the ‘psychological’ stress is robust (P < 0.05), and is not influenced by icv administration of either saline or CRH receptor antagonists including the selective CRF1 blocker NBI 30775 or the general blocker α-helical CRH. (d–f) Infusion of CRF1 antagonists block Fos expression in CA3. (d) At 60 min after stress termination, Fos is strongly expressed in CA3 of the P18 rat that had received icv saline 30 min prior to stress onset. In contrast, stress-induced Fos expression is practically abrogated by infusion of NBI 30775 (e) or α-helical CRH (f), 30 min prior to stress onset. Scale bar = 720 μm (a–f) and 80 μm (inset in b).
Figure 5.
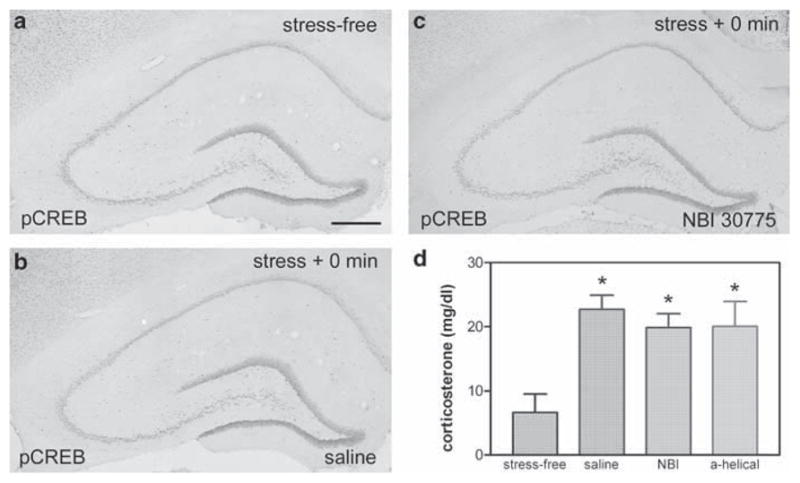
Restraint stress does not induce pCREB expression in adult hippocampus. (a) Unlike the immature hippocampus, numerous stress-free adult hippocampal neurons express pCREB. (b) A 30 min restraint stress does not influence this pCREB expression in saline-preinfused controls. (c) CRF1 antagonists prior to stress also have no significant effect on pCREB expression (see Figure 1 for the time-plan of antagonist infusions). (d) The restraint is a significant stress, leading to an increase in plasma corticosterone levels, and this stress-evoked glucocorticoid secretion is not influenced by icv infusion of saline, NBI 30775 or α-helical CRH, suggesting that central infusion of CRF1 antagonists does not block the peripheral stress response. Scale bar = 720 μm (a–c).
Groups of stressed animals were killed at several time-points, to study the hippocampal neurons that are activated by the ‘psychological’ stress (Figure 1). This neuronal activation was measured as the phosphorylation of the cyclic AMP responsive element binding protein CREB (pCREB), or the induction of the immediate-early gene c-fos. Groups of stressed animals were deeply anesthetized at 0, 15, 30 or 60 min after stress exposure for pCREB evaluation, and at 0, 30, 60, 90 or 120 min for analysis of c-fos expression (n = 5 for each time-point; Figure 1). In further experiments designed to fully define the time course of putative CREB phosphorylation in adult rats, six rats were killed at 0, 10 or 20 min from the onset of restraint stress, and the brains processed for pCREB analysis as described below (Figure 6).
Figure 1.
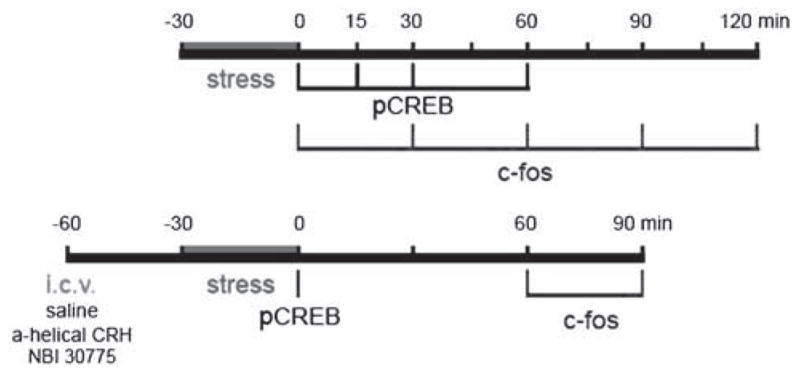
Schematic of the experimental plans used in the current study. The top bar shows the time course of evaluation of phosphorylated CREB (pCREB) and of Fos after the stress. The bottom bar indicates the schedule of administration of blockers of the corticotropin-releasing hormone (CRH) receptors.
Figure 6.
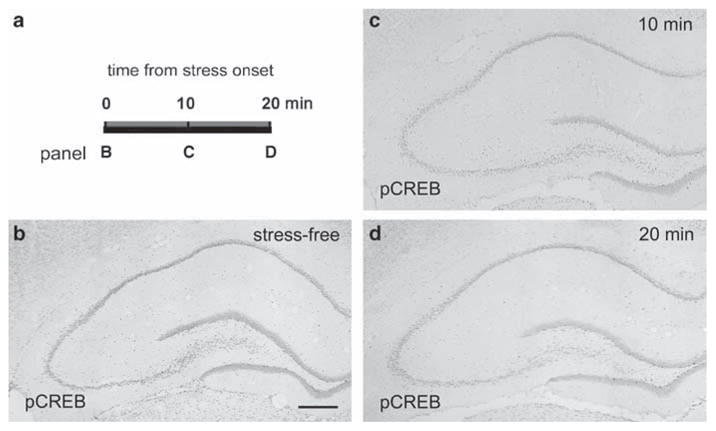
Restraint stress does not induce a rapid, transient pCREB expression in adult hippocampus. (a) Schematic of the experimental paradigm. In essence, pCREB was investigated at 10 and 20 min from the onset of the stress, to examine for a rapid and transient phosphorylation of the transcription factor. (b) In the stress-free adult hippocampus, many neurons express pCREB. (c) 10 min and, (d) 20 min after the onset of restraint stress, pCREB expression is not appreciably different from the stress-free pattern and extent. Scale bar = 720 μm (b–d).
To investigate the role of CRH in the activation of hippocampal neurons by stress, groups of either immature or adult rats (n = 4–8) were infused with either a general CRH receptor blocker, [9–41]-α-helical CRH, or a selective CRF1 receptor antagonist, NBI 30775. Both compounds were infused into the lateral ventricle (icv), at doses of 15 μg in 1 μl, via cannulae implanted 6–7 days earlier under halothane anesthesia, as described previously.56 After 30 min, the infused animals were subjected to the 30 min stress, then harvested immediately (for pCREB), or at 60 or 90 min later (for Fos), as shown in Figure 1.
Tissue handling and immunocytochemistry (ICC)
Brains from animals perfused using fresh 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB, pH 7.4) were sectioned coronally into 20 μm thick slices using a cryostat, and ICC was performed on free-floating sections using the avidin–biotin complex.23 Briefly, after treatment in 0.3% H2O2/PBS-T (0.01 M PB-saline containing 0.3% Triton X-100, pH 7.4) and blockade of nonspecific sites with 5% normal serum, sections were incubated in the primary antisera for 36 h at 4°C. The antisera included rabbit anti-pCREB (1:4000, Upstate Biotechnology, Lake Placid, NY, USA), rabbit anti-Fos (1:40 000, Oncogene, Ab-5, PC 38), or goat anti-CRF1 (1:10 000, Santa Cruz, CA, USA). After washes in PBS-T (3 × 5 min), sections were incubated in biotinylated goat-anti-rabbit IgG (for pCREB and Fos, 1:200, Vector laboratories, Burlingame, CA, USA), or biotinylated rabbit- anti-goat IgG (for CRF1) for 1 h, followed by the avidin–biotin–peroxidase complex solution (1:100, Vector) for 2 h. The reaction product was visualized by incubating the sections in 0.04% 3,3′-diamino-benzidine (DAB) containing 0.01% H2O2.
Double-labeling ICC
Sections were processed for concurrent immunolabeling of CRF1 and pCREB or Fos, as described.23 Briefly, sections were first incubated with goat anti-CRF1 (1:10 000), yielding a diffuse brown DAB reaction product. Sections were then exposed to rabbit anti-pCREB (1:4000) or anti-Fos (1:40 000), followed by the biotinylated second antibody and the avidin–biotin–peroxidase complex solutions as described above. To visualize pCREB or Fos, sections were rinsed, transferred to 0.01 M PB (pH 6.6), then incubated in a buffer containing 0.025% sodium nitroprusside and 0.01–0.02% benzidine dihydrochloride (BDHC) for 5–10 min. The granular blue deposits were visualized by immersing the sections in fresh incubation solution containing 0.003% H2O2 (3 min).
The pCREB antiserum was generated against an epitope consisting of the phosphopeptide (including Ser133) portion of pCREB, and does not recognize non-phosphorylated CREB. The specificity of CRF1 antiserum has been described.57
Statistical analysis
The effect of stress on corticosterone was determined using the Student’s t-test with significance levels set at P < 0.05.
Results
In immature rat hippocampus, ‘psychological stress’ induces both pCREB and Fos
A single 30 min ‘psychological stress’ resulted in a robust pCREB expression in hippocampal CA3, CA1 and the dentate gyrus (DG) in P18 rats (Figure 2). In stress-free animals, pCREB was virtually absent in the pyramidal cell layers (Figure 2a), but was selectively expressed in the inner portion of DG granule cell layer (GCL) labeling immature granule cells, as previously described (Figure 2e; Bender et al.58). These immature granule cells do not express the CRF receptor CRF1 (Figure 2e and f), and the stress-independent expression of pCREB within these neurons delineates a postmitotic, not fully differentiated stage of their maturation.58 At the termination of the 30-min stress period, robust pCREB expression was evident in CA3 and CA1 pyramidal cell layers (Figure 2b). This CREB phosphorylation reached its maximum at 15–30 min (Figure 2c and d). The signal was drastically diminished by 60 min after the end of stress. As shown in the 15 min group (Figure 2f and g), stress-induced pCREB expression was largely confined to CRF1-bearing neurons.
Figure 2.
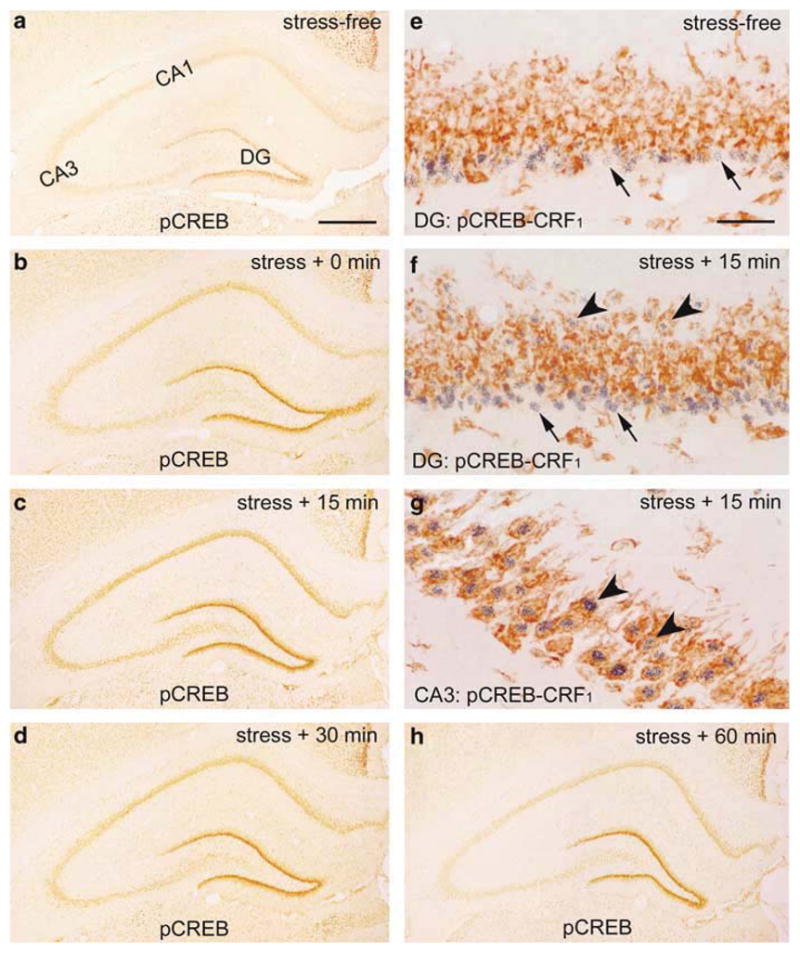
Stress induces pCREB expression in immature (postnatal day [P]18) rat hippocampus. (a) In the stress-free control, pCREB expression is not detected in CA3, though it is readily visible in the inner GCL, occupied by immature granule cells, within the dentate gyrus (DG). These immature granule cells (arrows in (e), also in (f), blue) do not express the CRH receptor CRF1 (brown). (b–d, h) The time course of pCREB expression induced by 30 min ‘psychological stress’. pCREB expression is detected strongly within CA3 pyramidal cell layer, where it peaks at 15 min after the termination of stress (45 min from its onset; (c)). pCREB is generally confined to CRF1-bearing cells (arrowheads in (f, g)). Scale bars = 720 μm (a–d, h) and 60 μm (e–g).
The ‘psychological stress’ also resulted in a strong Fos expression in hippocampal CA3 (Figure 3). Fos expression was not detected in stress-free immature hippocampus (Figure 3a). Stress-induced Fos expression was reliably detected in CA3 pyramidal cell layer and occasionally in CA1 at 30 min after stress termination (1 h after stress onset), and reached its maximal intensity and density at 60 min after stress termination (Figure 3d), then diminished progressively (Figure 3f). As shown in the higher magnification photomicrograph double-labeled for Fos and CRF1, stress-induced Fos expression in CA3 was most prominent in CRF1-expressing neurons (Figure 3e). Note that only a subset of neurons within the principal cell layer expressed Fos, even at peak response, and little Fos expression occurred in the DG GCL (Figure 3d).
Figure 3.
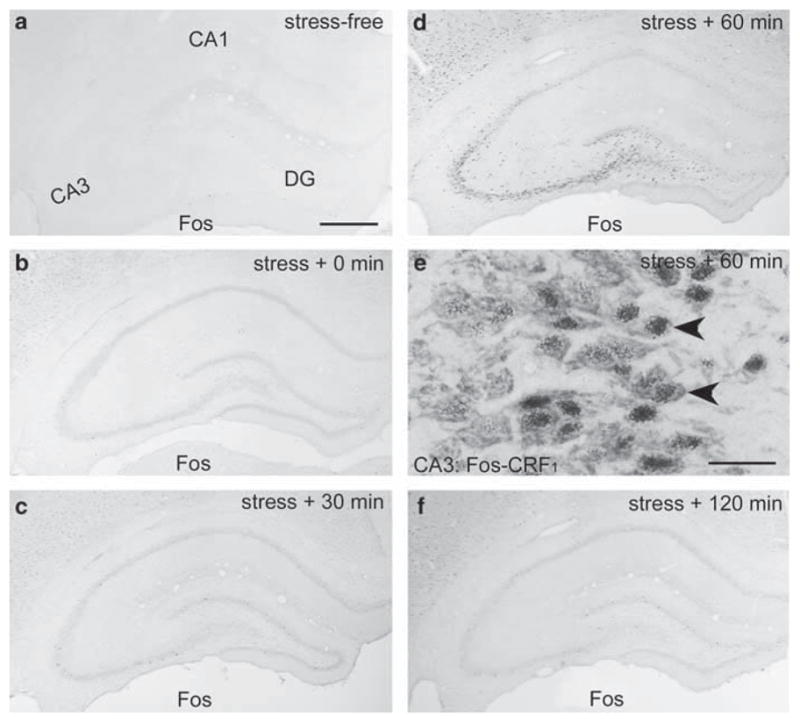
The time course of stress-induced Fos expression in P18 rat hippocampus. (a) Fos is not detected in the stress-free hippocampus. (b) At 30 min after the termination of ‘psychological stress’ (60 min from its onset), Fos expression is induced primarily in CA3. This expression peaks at 60 min after stress termination (d), and virtually disappears by 120 min (2.5 h from stress onset; (f)). As shown for the 60 min group (e), Fos-expressing CA3 pyramidal cells (blue immunoreactivity product) co-express CRF1 (brown, arrowheads). Scale bars = 720 μm (a–d, f) and 60 μm (e).
In immature hippocampus, CRF1 antagonists block stress-induced pCREB and Fos expression
To investigate whether hippocampal CRH was involved in stress-induced neuronal activation in this structure, we infused a general (α-helical CRH) or a CRF1-selective (NBI 30775) CRH receptor antagonists into left cerebral ventricle (icv) 30 min prior to the onset of stress, then examined the patterns of pCREB and Fos expression. To exclude the possibility that the antagonists diffused systemically and inhibited pituitary CRH receptors (and hence, the stress response) plasma corticosterone levels were analyzed.
As shown in Figure 4c, the acute psychological stress robustly elevated plasma corticosterone levels in both saline-infused and CRF1 antagonist-infused rats, compared with stress-free controls (P < 0.05). This indicates that the icv administration of the CRF1 antagonists did not interfere with the systemic response to the stress. However, stress-induced pCREB as well as Fos expression was largely abrogated in CRF1-antagonist treated animals. Comparing Figure 4a and b demonstrates a striking reduction of pCREB signal in the pyramidal cell layers in CA3 and CA1, as well as in the stress-sensitive, mature granule cells of DG, which are located within the outer portion of the GCL. The stress-independent expression of pCREB in the inner layer of immature granule cells was minimally affected. Weak remaining signal is shown in the inset of Figure 4b. A similar response to blocking of CRH receptors occurred for Fos, with virtual elimination of the stress-evoked expression (Figure 4d and e). Data for the general CRH receptor blocker resembled those for the selective CRF1 antagonist, and are presented for the Fos expression only (Figure 4f). Taken together, these data indicate that activation of the CRF1 receptor is required for stress-induced neuronal activation in immature hippocampus by central (likely hippocampal59) CRH.
In mature hippocampus, ‘psychological’ restraint stress induces Fos, but not pCREB expression, that is blocked by CRF1 antagonist
Expression of pCREB expression was present constitutively within numerous hippocampal neurons even in the ‘stress-free’ 3-month-old rat (Figure 5a). These included both principal cells and interneurons, as well as many granule cells. Exposure to a 30-min restraint stress did not influence pCREB expression appreciably. Not surprisingly, infusion of CRH receptor blockers or saline vehicle to stressed rats also led to no discernible alteration of pCREB expression (Figure 5b and c). Analysis of plasma corticosterone levels confirmed that the restraint stress elicited a robust hormonal response, and, as in the immature rat, affirmed that central administration of the antagonists did not abolish the peripheral hormonal response to stress (Figure 5d).
To consider the possibility that the time course of pCREB expression in the adult hippocampus in response to stress was more rapid than that observed for the juvenile hippocampus, groups of animals were evaluated also at early time-points after the onset of the restraint stress, that is, 10 and 20 min (Figure 6a). As shown in Figure 6, pCREB was not enhanced above the stress-free levels at any of the early time-points evaluated. These data do not support the possibility that stress leads to a rapid, transient pCREB expression in hippocampal principal cell layers.
These data raised the possibility that a 30-min restraint stress, though sufficient to provoke a hormonal stress response, does not engage the hippocampal formation of mature rats. To evaluate this possibility, Fos expression was examined in adult animals exposed to this stress. This immediate-early gene was strongly induced by the stress (Figure 7), but with a pattern distinct from that in the immature hippocampus (see Figure 3). Fos expression in adult occurred primarily in CA1. A few Fos-positive cells were visible already at the termination of stress (inset, Figure 7b). However, robust Fos expression in CA1 was present at 90 min after the termination of the stress (Figure 7e), when a weak signal was found in the CA3 pyramidal cell layer as well. Fos expression was confined primarily to CRF1 expressing neurons in CA1 (Figure 7h), and was abolished by infusion of CRF1 antagonist NBI 30775 30 min prior to stress onset (Figure 7i).
Figure 7.
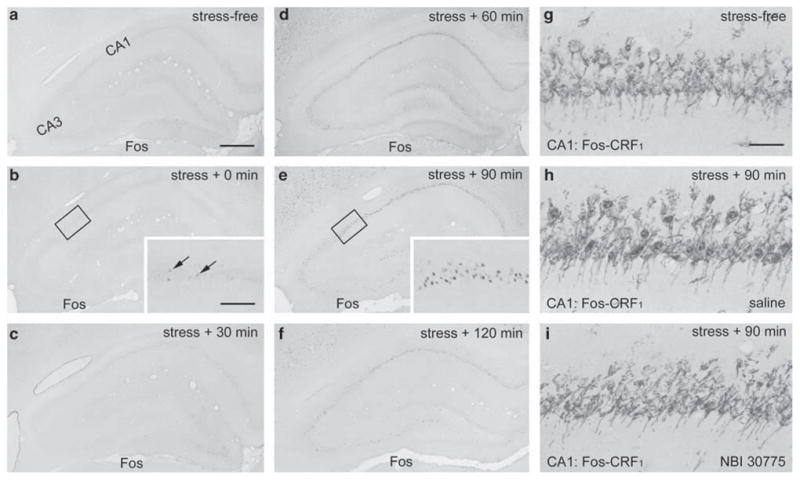
Restraint stress induces Fos expression in adult hippocampal CA1, which can be blocked by prior infusion of CRF1 antagonists. (a–f) Time course of Fos expression in adult hippocampus induced by 30-min restraint stress. (b, c) Rare Fos expressing neurons are apparent in the CA1 pyramidal cell layer immediately and 30 min after the termination of the stress (arrows in inset, b). (d, e) Strong Fos expression is detected in CA1 60 and 90 min after the stress. At these time-points, Fos is also expressed to a lesser extent in CA3. (g–i) CA1 neurons double-labeled for Fos and CRH receptor CRF1: Fos is not expressed in CRF1-expressing neurons of control animals implanted with cannula 6 days earlier (g). Stress-induced Fos expression (blue immunoreaction product) is co-localized with CRF1 (brown) and is not abolished by saline pre-administration through the preimplanted cannula (h, see Figure 1 for schedule). Prior infusion of the CRF1 antagonist NBI 30775 blocks restraint-induced Fos expression in CA1 (i). Scale bar = 720 μm (a–f), 180 μm (insets in b, c, e) and 60 μm (g–i).
Discussion
The major findings of this study are: (1) Hippocampal neurons are activated during ‘emotional’ stress, such as restraint, in both immature and adult rat. (2) Phosphorylation of CREB occurs in immature – but not in adult – hippocampus, in response to acute restraint stress. (3) Fos expression delineates activated hippocampal neurons at both ages, but the cell populations involved overlap only partially. (4) Stress-evoked neuronal activation in hippocampus is eliminated by pre-treatment with general or selective CRH receptor antagonists in both immature and adult rat. Taken together, these data suggest that stress engages different mechanisms to influence immature and adult hippocampus, though at both ages CRH receptors are involved. In particular, selective, robust phosphorylation of the transcription factor CREB by stress in developing hippocampus may herald initiation of transcriptional events that contribute to the enduring effects of early-life stress on hippocampal function.
Whether stress influences hippocampal function and structure is an important question to human health for several reasons. First, correlational and epidemiological studies have implicated stress in human disorders that involve the hippocampus, including dementia,60,61 depression,62,63 and schizophrenia.13 In addition, genetic predisposition accounts for only a fraction of diseases – such as Alzheimer’s – where hippocampal dysfunction is profound.64,65 The possibility that experience, including stress, may contribute to human neurodegenerative disorders associated with major hippocampal dysfunction has therefore received much interest. In addition, early-life stress may impact the hippocampus to a greater degree than stress later in life.2,4,9 For example, early-life neglect or abuse have been correlated with reduced hippocampal volume,66 as well as reduced cognitive function67 that may emerge later in life.61 However, human studies, including those cited here, are correlational, posing the difficulty of dissecting out the specific effects of stress from those of genetic background and many other confounding variables.
Using animal models, profound effect of acute (as well as chronic) stress on the function of adult hippocampus have been described (e.g. Kerr et al.68 and Luine et al.69). These studies demonstrated stress-evoked activation of immediate early gene expression, as well as electrophysiological and behavioral changes (e.g. Kim and Diamond,30 Pavlides et al.,70 Alfarez et al.71 and Blank et al.72). Patterns of Fos expression after stress in adult hippocampus have been described by several groups at both mRNA and protein level,73–76 and others have distinguished the effects of novelty from those of stress per se.77,78 The effects of stress on hippocampal neurons during development have been less studied.79 This is somewhat surprising, because both human and animal model studies suggest that the consequences of early-life stress on the function and structure of the hippocampus might differ significantly depending on the age at which the stress is experienced.
Here, we find both important commonalities as well as interesting differences between pCREB and Fos expression in hippocampal neurons in response to stress. The significance of these differences merits discussion. First, both mature and juvenile (P18) hippocampal neurons were activated in response to acute stress, suggesting that this structure is clearly within the ‘circuit’ involved in perception and response to acute stressful situations. The stresses used here involved restraint and noise/restraint at both ages. Whereas is it possible that the results of the current studies might be stress-specific, the selective Fos induction in CA1 of adult rats is in agreement with those reported by other groups. For example, Cullinan et al.73 found a similar Fos induction in CA1 after both restraint and swim stresses, and Abraham and Kovacs80 distinguished the effects of ‘psychological’ restraint stress from that of a physical stressor, ether exposure, that did not activate the hippocampus. In addition, the possibility that the restraint is not perceived as stressful either in adult or in immature rats is unlikely, because at both ages this treatment led to robust, and comparable, increases of plasma glucocorticoids (Figures 4 and 5).
The molecular mechanisms by which stress activates hippocampal neurons are not fully understood. The relationship of pCREB and Fos, in particular, in the cascade of events initiated by cellular calcium entry has been a focus of intensive research (see Hardingham and Bading81 for review). In the context of the current work, Fos expression may be evoked by CREB-dependent and CREB-independent pathways, that, in turn, are governed by the route of calcium entry and other less well understood signals. The ability of stress to induce Fos expression, without pCREB changes, in adult hippocampus suggests that a pCREB-independent pathway is involved. However, the congruence of pCREB and Fos in the immature hippocampus does not necessarily imply that the former is causally involved (or ‘upstream’) of the latter. CREB phosphorylation may occur in addition to, and not necessarily as a pre-requisite for, Fos expression. Indeed, a general divergence of pCREB and Fos expression in response to sensory stimulation (‘experience’) in the immature rodent has recently been described.82
At both ages, blocking CRH receptors within the hippocampus, and without interference with systemic glucocorticoid release, abrogated the ability of the ‘psychological’ stress to activate hippocampal neurons. This, supported by the fact that many of the activated neurons in the hippocampal formation expressed the CRF1 receptor, points to a substantial role of CRH receptor activation in the transduction of stress signals within the hippocampus. Indeed, a role for CRH in stress-evoked neuronal changes has been suggested,40,43,56,83 in addition to the well established and robust effects of glucocorticoids.1,28 It should be noted that in the current study, we chose to use Fos and pCREB as markers of neuronal activation. These are likely to respond to CRH, because they involve cAMP-mediated mechanisms, which are likely to be induced by activation of the G-protein coupled CRH receptor CRF1.37 This study was not designed to consider all of the potential molecular cascades that might be evoked by stress, including those related to GR and MR binding by glucocorticoids. The latter exert genomic and nongenomic effects on a wide spectrum of molecules, eventually leading to electrophysiological, functional and structural alteration of neurons.1,29,30 These changes also largely occur at a longer timescale, and whether these GR/MR mediated changes are also age-selective is a topic that deserves further study. Here we considered relatively rapid activation at the minute-to-hour timescale, of hippocampal neurons by acute stress, and relied on molecular processes that are readily detectable.73,84
The current study finds that different neuronal populations express c-fos in response to stress in adult vs juvenile hippocampus. The potential functional consequence of these findings is not immediately clear. Electrophysiological studies suggest that stress alters synaptic plasticity in both CA3 and CA1, but the responsible mechanisms are extremely complex and involve pre- and postsynaptic elements in both regions.22,30,70 The absence of Fos activation in CA3 in adult neurons is particularly notable, and it is tempting to speculate that this might be a neuroprotective mechanism: Stress35 as well as large doses of CRH may injure and kill hippocampal CA3 neurons in developing hippocampus.49,85 The absence of this activation in the adult might prevent hyper-excitability and excitotoxicity of this neuronal population.
Finally, the age-selective activation of pCREB in immature hippocampus is intriguing. Stress early in life influences hippocampal function in an enduring manner. During early postnatal life, psychological stress, including recurrent separation86 interferes with learning and memory later in life. More recently, longer psychological stress during the first/second week of life has been shown to abolish long-term potentiation and provoke impaired learning and memory during middle age.22 This was associated with altered expression of several hippocampal genes, including the gene for CRH itself (Brunson et al.56 and unpublished data). Remarkably, the CRH gene is regulated by CREB, via a CRE in its promoter.87 Thus, stress-evoked CREB phosphorylation may set in motion a program of altered expression of CRE-regulated genes, which might influence the structure and function of the hippocampus long-term. Whether this is indeed the case will require future studies.
In summary, acute ‘psychological stress’ engages the hippocampus in both juvenile and adult rat, leading to age-specific patterns of neuronal activation and distinct cascades of intracellular events. The phosphorylation of the transcription factor CREB selectively in the developing hippocampus suggests a mechanism for the long-lasting effects of early-life stress on the hippocampal formation.
Acknowledgments
We thank Michele Hinojosa for excellent editorial assistance. This work was supported in part by NIH NS28912, MH73136 (TZB) and the NARSAD foundation young investigator award (YC).
References
- 1.McEwen B. Stress and hippocampal plasticity. Ann Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez M, Ladd C, Plotsky P. Early adverse experience as a developmental risk factor for later psychopathology evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 3.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids, and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 4.Avishai-Eliner S, Brunson K, Sandman C, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Miller DB, O’Callaghan JP. Aging, stress and the hippocampus. Ageing Res Rev. 2005;4:123–140. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Front Neuroendocrinol. 2006 doi: 10.1016/j.yfrne.2006.02.001. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Prog Brain Res. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- 9.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin- releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 10.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez DM. Stress and the developing limbic–hypothalamic– pituitary–adrenal axis. Psychoneuroendocrinology. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 12.Walker CD, Toufexis DJ, Burlet A. Hypothalamic and limbic expression of CRF and vasopressin during lactation: implications for the control of ACTH secretion and stress hyporesponsiveness. Prog Brain Res. 2001;133:99–110. doi: 10.1016/s0079-6123(01)33008-x. [DOI] [PubMed] [Google Scholar]
- 13.Benes FM. The role of stress and dopamine–GABA interactions in the vulnerability for schizophrenia. J Psychiatr Res. 1997;31:257–275. doi: 10.1016/s0022-3956(96)00044-1. [DOI] [PubMed] [Google Scholar]
- 14.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 15.Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978;202:1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- 16.Hibberd C, Yau JL, Seckl JR. Glucocorticoids and the ageing hippocampus. J Anat. 2000;197:553–562. doi: 10.1046/j.1469-7580.2000.19740553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols NR, Zieba M, Bye N. Do glucocorticoids contribute to brain aging? Brain Res Brain Res Rev. 2001;37:273–286. doi: 10.1016/s0165-0173(01)00131-x. [DOI] [PubMed] [Google Scholar]
- 18.Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- 19.Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaral D, Dent J. Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopy study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 21.Henze D, Urban N, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 22.Brunson KL, Kramar E, Lin B, Chen Y, Colgin L, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Bender R, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatio-temporal analysis. J Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilang-Bleuel A, Rech J, De Carli S, Holsboer F, Reul JM. Forced swimming evokes a biphasic response in CREB phosphorylation in extrahypothalamic limbic and neocortical brain structures in the rat. Eur J Neurosci. 2002;15:1048–1060. doi: 10.1046/j.1460-9568.2002.01934.x. [DOI] [PubMed] [Google Scholar]
- 25.Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grino M, Paulmyer-Lacroix O, Anglade G, Oliver C. Molecular aspects of the regulation of the hypothalamo-pituitary-adrenal axis during development in the rat. Ann NY Acad Sci. 1995;771:339–351. doi: 10.1111/j.1749-6632.1995.tb44693.x. [DOI] [PubMed] [Google Scholar]
- 27.Dent GW, Okimoto DK, Smith MA, Levine S. Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology. 2000;71:333–342. doi: 10.1159/000054554. [DOI] [PubMed] [Google Scholar]
- 28.de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 29.Joels M. Modulatory actions of steroid hormones and neuropeptides on electrical activity in brain. Eur J Pharmacol. 2000;405:207–216. doi: 10.1016/s0014-2999(00)00554-9. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Diamond D. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 31.Magarinos A, McEwen B. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- 32.Magarinos A, McEwen B. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 33.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dachir S, Kadar T, Robinzon B, Levy A. Cognitive deficits induced in young rats by long-term corticosterone administration. Behav Neural Biol. 1993;60:103–109. doi: 10.1016/0163-1047(93)90173-f. [DOI] [PubMed] [Google Scholar]
- 35.Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 36.Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behan DP, Grigoriadis DE, Lovenberg T, Chalmers D, Heinrichs S, Liaw C, et al. Neurobiology of corticotropin releasing factor (CRF) receptors and CRF-binding protein: implications for the treatment of CNS disorders. Mol Psychiatry. 1996;1:265–2177. [PubMed] [Google Scholar]
- 38.Negrao AB, Licinio J. Stress-responsive neuropeptides in major depression. Mol Psychiatry. 1996;1:300–301. [PubMed] [Google Scholar]
- 39.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 40.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 41.Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, et al. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;39:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 42.Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol Psychiatry. 2001;6:647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakshi VP, Kalin NH. Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry. 2000;48:1175–1198. doi: 10.1016/s0006-3223(00)01082-9. [DOI] [PubMed] [Google Scholar]
- 45.Jochman KA, Newman SM, Kalin NH, Bakshi VP. Corticotropinreleasing factor-1 receptors in the basolateral amygdala mediate stress-induced anorexia. Behav Neurosci. 2005;119:1448–1458. doi: 10.1037/0735-7044.119.6.1448. [DOI] [PubMed] [Google Scholar]
- 46.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, et al. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;14:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 49.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: preand postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 53.Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Refojo D, Echenique C, Muller MB, Reul JM, Deussing JM, Wurst W, et al. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc Natl Acad Sci USA. 2005;102:6183–6188. doi: 10.1073/pnas.0502070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatalski CG, Guirguis C, Baram TZ. Corticotropin-releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunson K, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Longterm, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Brunson K, Müller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF1)-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bender RA, Lauterborn JC, Gall CM, Cariaga W, Baram TZ. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur J Neurosci. 2001;13:679–686. doi: 10.1046/j.1460-9568.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Bender RA, Brunson KL, Pomper JL, Grigoriadis DE, Wurst W, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci USA. 2004;101:15782–157827. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer JS, Terayama Y, Konno S, Akiyama H, Margishvili GM, Mortel KF. Risk factors for cerebral degenerative changes and dementia. Eur Neurol. 1998;39(Suppl 1):7–16. doi: 10.1159/000052064. [DOI] [PubMed] [Google Scholar]
- 61.Kaplan Z, Iancu I, Bodner E. A review of psychological debriefing after extreme stress. Psychiatr Serv. 2001;52:824–827. doi: 10.1176/appi.ps.52.6.824. [DOI] [PubMed] [Google Scholar]
- 62.Gold PW, Wong ML, Chrousos GP, Licinio J. Stress system abnormalities in melancholic and atypical depression: molecular, pathophysiological, and therapeutic implications. Mol Psychiatry. 1996;1:257–264. [PubMed] [Google Scholar]
- 63.Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- 64.Mattson MP, Chan SL. Dysregulation of cellular calcium homeostasis in Alzheimer’s disease: bad genes and bad habits. J Mol Neurosci. 2001;17:205–224. doi: 10.1385/JMN:17:2:205. [DOI] [PubMed] [Google Scholar]
- 65.Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 66.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse – a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ammerman R, Cassisi J, Hersen M, Hasselt VV. Consequences of physical abuse and neglect in children. Clin Psychol Rev. 1986;6:291–310. [Google Scholar]
- 68.Kerr DS, Campbell LW, Applegate MD, Brodish A, Landfield PW. Chronic stress-induced acceleration of electrophysiologic and morphometric biomarkers of hippocampal aging. J Neurosci. 1991;11:1316–1324. doi: 10.1523/JNEUROSCI.11-05-01316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luine V, Villegas M, Martinez C, McEwen B. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 70.Pavlides C, Nivon L, McEwen B. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 71.Alfarez D, Joels M, Krugers H. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- 72.Blank T, Nijholt I, Spiess J. Molecular determinants mediating effects of acute stress on hippocampus-dependent synaptic plasticity and learning. Mol Neurobiol. 2004;29:131–138. doi: 10.1385/MN:29:2:131. [DOI] [PubMed] [Google Scholar]
- 73.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 74.Kovács KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 75.Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in ‘neurogenic’ stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;393:244–266. [PubMed] [Google Scholar]
- 76.Ons S, Marti O, Armario A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. J Neurochem. 2004;89:1111–1118. doi: 10.1111/j.1471-4159.2004.02396.x. [DOI] [PubMed] [Google Scholar]
- 77.Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol. 1999;38:234–246. [PubMed] [Google Scholar]
- 78.Pace TW, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer RL. Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur J Neurosci. 2005;22:1679–1690. doi: 10.1111/j.1460-9568.2005.04354.x. [DOI] [PubMed] [Google Scholar]
- 79.Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abraham IM, Kovacs KJ. Postnatal handling alters the activation of stress-related neuronal circuitries. Eur J Neurosci. 2000;12:3003–3014. doi: 10.1046/j.1460-9568.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- 81.Hardingham GE, Bading H. Calcium as a versatile second messenger in the control of gene expression. Microsc Res Tech. 1999;46:348–355. doi: 10.1002/(SICI)1097-0029(19990915)46:6<348::AID-JEMT3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 82.Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic- pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci. 2006;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 84.Kovács KJ, Sawchenko PE. Sequence of stress induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huot R, Plotsky P, Lenox R, McNamara R. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- 87.Seasholtz AF, Thompson RC, Douglass JO. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988;2:1311–1319. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]


