Figure 8. Model of caveolae facilitating Ang II-induced vascular BK channel dysfunction in diabetes.
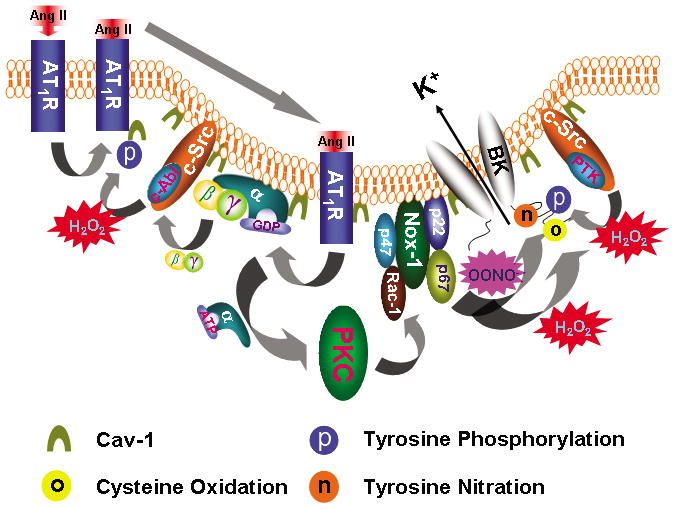
Upon Ang II stimulation, AT1R translocates into caveolae, which requires c-Abl-mediated cav-1 tyrosine 14 phosphorylation. In caveolae, AT1R interacts with Gαq that activates the PKC and enhances NOX-1 complex activity through p47phox phosphorylation and Rac-1 phosphorylation. Binding of Ang II to AT1R releases Gβγ which activates c-Src/c-Abl, resulting in increase of cav-1 tyrosine 14 phosphorylation and AT1R translocation. ROS enhances c-Src/protein tyrosine kinase (PTK) activity and inhibits protein tyrosine phosphatase (PTP) activity, which leads to increase in protein tyrosine phosphorylation. Both H2O2 and OONO− directly cause BK channel cysteine oxidation and OONO− produces BK channel tyrosine nitration. These mechanisms of Ang II-induced oxidative post-translational modification impair vascular BK channel function.
