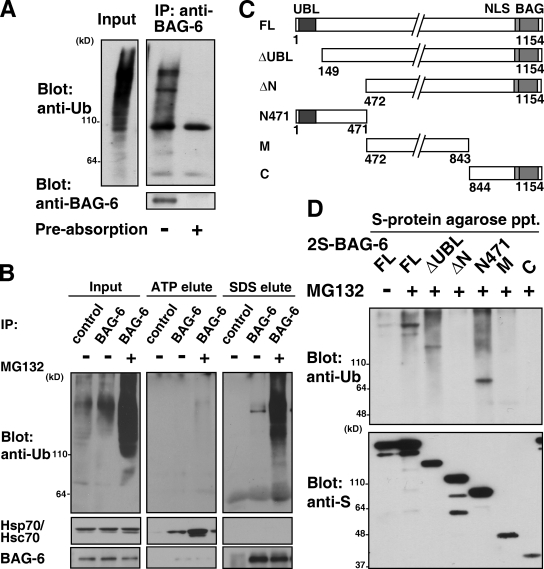
Figure 2.
BAG-6 associates with polyubiquitinated proteasomal substrates. (A) Endogenous BAG-6 protein was affinity purified from extracts of MG132-treated HeLa cells with an antibody against BAG-6, and the precipitates were immunoblotted with antibodies against ubiquitin and BAG-6. Ubiquitin coprecipitation of BAG-6 was abolished by preabsorption with an excess of BAG-6 antigen. (B) Endogenous BAG-6 protein precipitated from HeLa cell extracts was treated with 5 mM ATP (ATP-elute) before boiling in 1% SDS (SDS-elute). Each eluate was immunoblotted with antibodies against ubiquitin, Hsp70/Hsc70, and BAG-6. Cells were treated with (+) or without (−) 20 µM MG132 for 6 h before being harvested as indicated. Immunoglobulin derived from nonimmune rabbit serum was used in immunoprecipitation as a negative control. (C) Schematic representation of the BAG-6 deletion mutant proteins used in this study. The numbers denote corresponding amino acid numbers. (D) N-terminal 471 amino acids of BAG-6 were essential for the binding with polyubiquitinated proteins. The full-length (FL) form of 2S-tagged BAG-6 and its truncated derivatives were expressed in HeLa cells as indicated. Before harvesting, cells were treated with or without 10 µM MG132 for 12 h. Each form of BAG-6 was affinity purified with S-protein agarose, and the bound materials were blotted with antibodies against ubiquitin and S peptide.