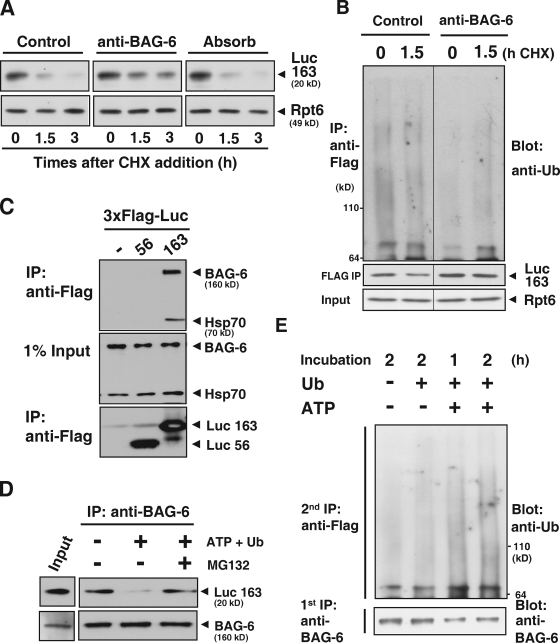
Figure 5.
BAG-6 provides a platform that is necessary for linking puromycin-labeled defective protein with degradation machinery. (A) Addition of antibody against BAG-6 inhibited the degradation of puromycin-labeled, truncated luciferase in vitro. A messenger RNA encoding the 3xFlag-tagged N-terminal 163 residues of luciferase (Luc163) was incubated in a rabbit reticulocyte lysate chasing with 2 mM puromycin addition. After addition of 50 µg/ml anti–BAG-6 antibody, cycloheximide (CHX) chase analysis was performed, and lysates were harvested at the indicated times and probed with antibodies against Flag and Rpt6 to evaluate the stability of puromycin-labeled Luc163 (Luc163). Non-immune rabbit IgG or antibody against BAG-6 that was preabsorbed with an excess amount of recombinant antigen was used as a negative control. (B) Addition of antibody against BAG-6 inhibited ubiquitination of puromycin-labeled, truncated luciferase. Blot with Flag-Luc163 and Rpt6 are indicated as loading controls. (C) Immunoprecipitation of in vitro translated Luc163 with anti-Flag M2 beads coprecipitated endogenous BAG-6 and Hsp70 from lysates. (D) BAG-6 provided a platform for the targeted degradation of Luc163. Endogenous BAG-6 was immunoprecipitated from a rabbit reticulocyte lysate that was translating 3xFlag-tagged Luc163. The precipitated immunocomplex was further incubated in the presence (+) or absence (−) of 5 mM ATP, ubiquitin, and 25 µM MG132 at 37°C for 2 h as indicated. After incubation, the precipitated complexes were subjected to Western blot analysis with an antibody against Flag to examine the stability of Luc163 on BAG-6 during the incubation periods. (E) Endogenous BAG-6 was immunoprecipitated (first IP) as in D, and the precipitated complex was incubated with MG132 under the conditions indicated. After incubation, the complexes were denatured by SDS and diluted samples were further subjected to precipitation with anti-Flag M2 agarose (second IP). Precipitated, Flag-tagged Luc163 was blotted with antibody against ubiquitin to estimate the extent of its modification with ubiquitin.