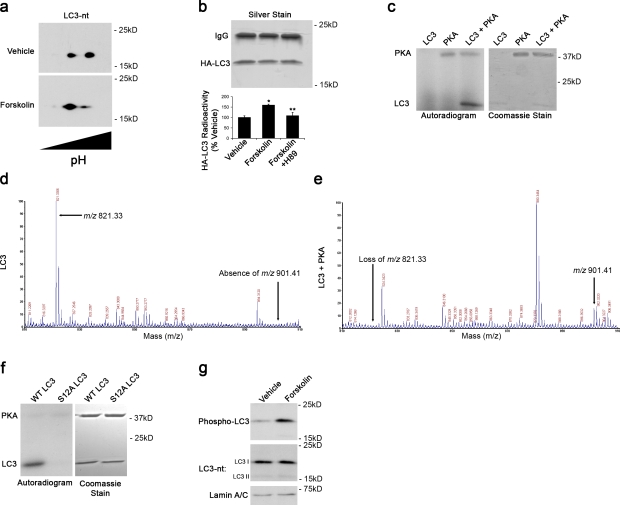
Figure 3.
PKA directly phosphorylates LC3. (a) 2D immunoblot probed for LC3 shows distinct species differing by isoelectric points. Forskolin increases abundance of species with a more acidic isoelectric point. (b) Metabolic labeling reveals greater 32P incorporation in HA-LC3 immunoprecipitated from forskolin-treated cells compared with control cells. Error bars indicate mean ± SEM. *, P < 0.05 versus vehicle; **, P < 0.05 versus forskolin (n = 3 independent experiments). (c) In vitro kinase assay with recombinant PKA and purified rat LC3. Phosphorylation was detected by autoradiography. Coomassie stain shows migration of the recombinant proteins. (d and e) Recombinant LC3 was incubated with ATP in the presence and absence of PKA. In the presence of PKA and ATP, the m/z 821.33 ion is lost with the appearance of an m/z 901.41 ion. (f) Mutation of the LC3 phosphorylation site to S12A abolishes PKA phosphorylation of LC3. (g) Immunoblot of cortical neurons treated with forskolin or DMSO (vehicle) probed with phospho-specific LC3 antibody, total LC3 antibody (LC3-nt), and lamin antibody as a loading control.