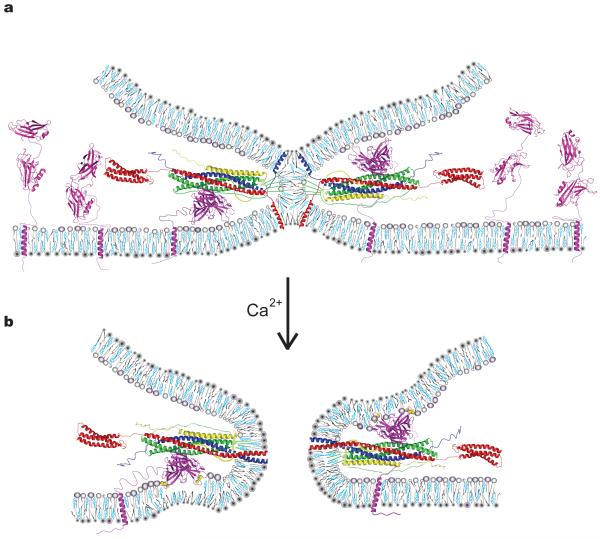
Figure 6.
Model of a general Ca2+–SNARE–synaptotagmin fusion-triggering mechanism. (a) Membrane stalk induced by SNARE complex formation prior to Ca2+ arrival between a vesicle (top membrane) and the plasma membrane (bottom membrane). All components are drawn to scale. For details about the generation of the model see Supplementary Methods. Both SNARE-bound and uncomplexed Syt3 molecules residing in the plasma membrane are shown. The model also applies to Syt1 except that the transmembrane domain of Syt1 would reside in the synaptic vesicle. We drew SNARE-bound Syt3 poised to interact with either plasma or synaptic vesicle membrane; it is unknown to which membrane (or both) synaptotagmins bind. Note that in our model simultaneous binding of both complexin and synaptotagmin to SNARE complex is sterically permissible although (partial) competition has been observed in solution17 (syntaxin–red, synaptobrevin–blue; SNAP25-green; complexin-yellow; synaptotagmin-magenta). Phospholipids are shown as gray cartoons, cholesterol in cyan. (b) Fusion pore after Ca2+ (yellow spheres) binding to Syt3. The color code is identical to panel (a). Upon Ca2+-influx Syt3 Ca2+-binding loops coordinate Ca2+ with anionic phospholipids, penetrating the lipid headgroup region8, and may induce positive curvature10.