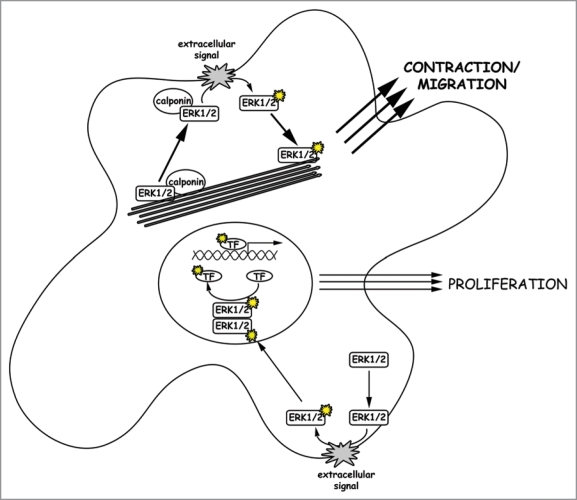
Figure 1.
Activation of different ERK1/2 populations. ERK1/2 translocation to the cell cortex, where ERK1/2 gets activated via the Raf/Ras/MEK signaling cascade, is initiated by an extracellular signal. We assume that two populations of ERK1/2 molecules exist in a cell. First there is free cytosolic ERK1/2, which translocates upon activation and dimerization into the nucleus to activate proliferation in response to extracellular growth signals. Secondly, there is an ERK1/2 population bound to both calponin and F-actin. Upon a migratory or contractile stimulus, this ERK1/2 population translocates together with its scaffold calponin to the cell cortex where it is activated. Upon activation, monomeric active ERK1/2 molecules move back to actin filaments where they trigger cell migration or other contractile events of the cell. In cells with a pronounced actin cytoskeleton, the latter pathway might be dominant due to attracting more ERK1/2 molecules to the actin filaments and thereby depleting the kinase from the nucleus.