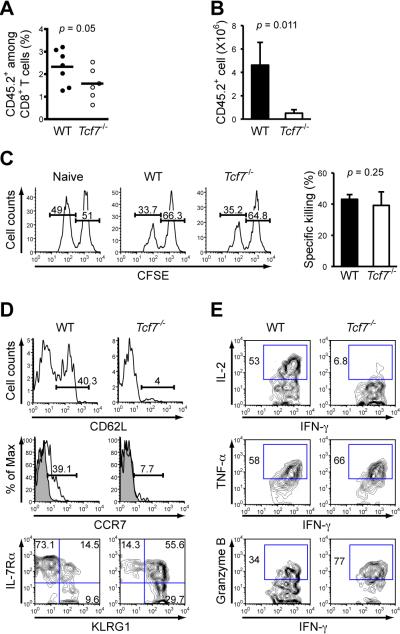
Figure 2. Tcf7-/- memory CD8+ T cells are impaired in secondary expansion and in differentiation to a Tcm phenotype.
(A) Frequency of early memory OT-I cells in PBLs. B6.SJL recipients of either WT or Tcf7-/- OT-I cells were infected with actA-LM-Ova, and the percentage of CD45.2+ OT-I in CD8+ T cells was determined on days 34-44 after infection. Data are pooled results from 2 independent experiments.
(B) Secondary expansion of memory CD8+ T cells in the spleens. Sorted WT or Tcf7-/- memory OT-I T cells (6 × 103) were transferred into naïve B6.SJL hosts, followed by infection with 2 × 105 CFU of virulent LM-Ova. The numbers of CD45.2+ OT-I cells in the spleens were determined 6 days later. Data are means ± s.d. (n = 3). Similar results were obtained when total splenocytes containing equivalent numbers of WT or Tcf7-/- memory OT-I cells were transferred without sorting separation (not shown).
(C) In vivo killing capacity of memory CD8+ T cells. CD45.2+ splenocytes were differentially labeled with CFSE. Ova peptide-pulsed CFSElo and non-peptide pulsed CFSEhi cells were injected at 1:1 ratio into naïve or immune chimeras (35 days after infection). The spleens were harvested 4 hrs later, and percentages of CFSElo and CFSEhi cells in CD45.2+ splenocytes were determined. Data are representative of 2 independent experiments with similar results.
(D) Surface staining for CD62L, CCR7, IL-7Rβ, and KLRG1 on antigen-specific memory CD8+ T cells. During days 75-85 after infection with actA-LM-Ova, splenocytes from WT or Tcf7-/- OT-I recipients were stained. Percentages of CD62Lhi and CCR7+ subsets were shown in histograms, with shaded histogram denoting isotype control. Data are representative of 2-3 independent experiments (n ≥ 4).
(E) Intracellular detection of IFN-γ, IL-2, TNF-α, and granzyme B in memory CD8+ T cells. Splenocytes were stimulated with Ova peptide for 6 hrs, followed by surface and intracellular staining. Gating of positive populations was based on respective isotype controls. All data are representative of 3 independent experiments (n ≥ 5).