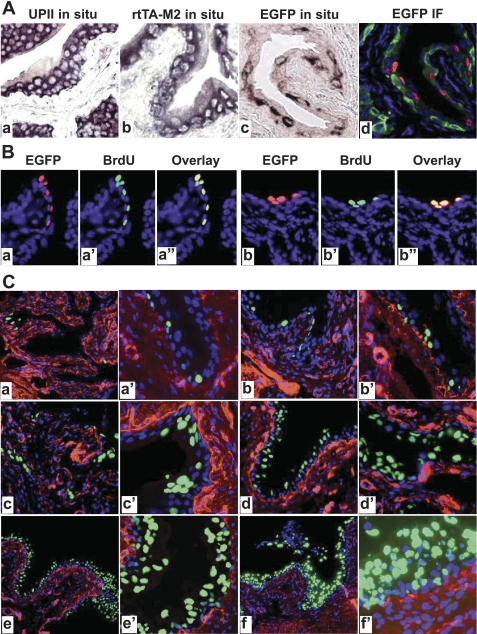
Fig. 4.
Correlation of EGFP expression with urothelial proliferation. A: paraffin sections from WT (a) and rtTA-M2/TRE-EGFP double transgenic mice treated with Dox for 10 days (b–d) were subject to in situ hybridization (a–c) or anti-EGFP immunofluorescent staining (d). While anti-sense probes for endogenous mouse UPII (a) and for rtTA-M2 (b) labeled urothelial cells almost uniformly, anti-sense probe for EGFP labeled urothelial cells selectively (c). An adjacent section to (c) stained with anti-EGFP (red) and counterstained with anti-keratin 5 (green) and DAPI (blue) confirmed the spotty expression of EGFP in the urothelial cells. B: colocalization of EGFP-expressing and 5-bromo-2-deoxyuridine (BrdU)-incorporating cells. Double transgenic mice harboring UPII-rtTA-M2 and TRE-EGFP transgenes were treated with Dox and BrdU simultaneously for 10 days and their bladders were paraffin-sectioned and stained with anti-EGFP (a and b; red), anti-BrdU (a′ and b′; green), and counterstained with DAPI (blue). The images of anti-EGFP and anti-BrdU staining were merged (a″ and b″; overlay). Note that EGFP-expressing cells overlapped precisely with BrdU-incorporating cells (yellow), suggesting that these cells were undergoing proliferation and represented newly divided cells. C: time course of Dox-induced EGFP expression in the UPII-rtTA-M2/TRE-EGFP transgenic mice. The double transgenic mice were treated with Dox for 3 days (a and a′), 10 days (b and b′), 20 days (c and c′), 40 days (d and d′), 90 days (e and e′), and 150 days (f and f′). The bladders from the Dox-treated mice were processed for frozen sections and antibody staining (anti-type IV collagen; red) and counterstaining (DAPI; blue). Note the increased EGFP expression with increased time of Dox induction. Magnifications: all panels in A and B: ×200; and in C: a, b, c, d, e, and f, ×50; a′, b′, c′, d′, e′, and f′, ×200.