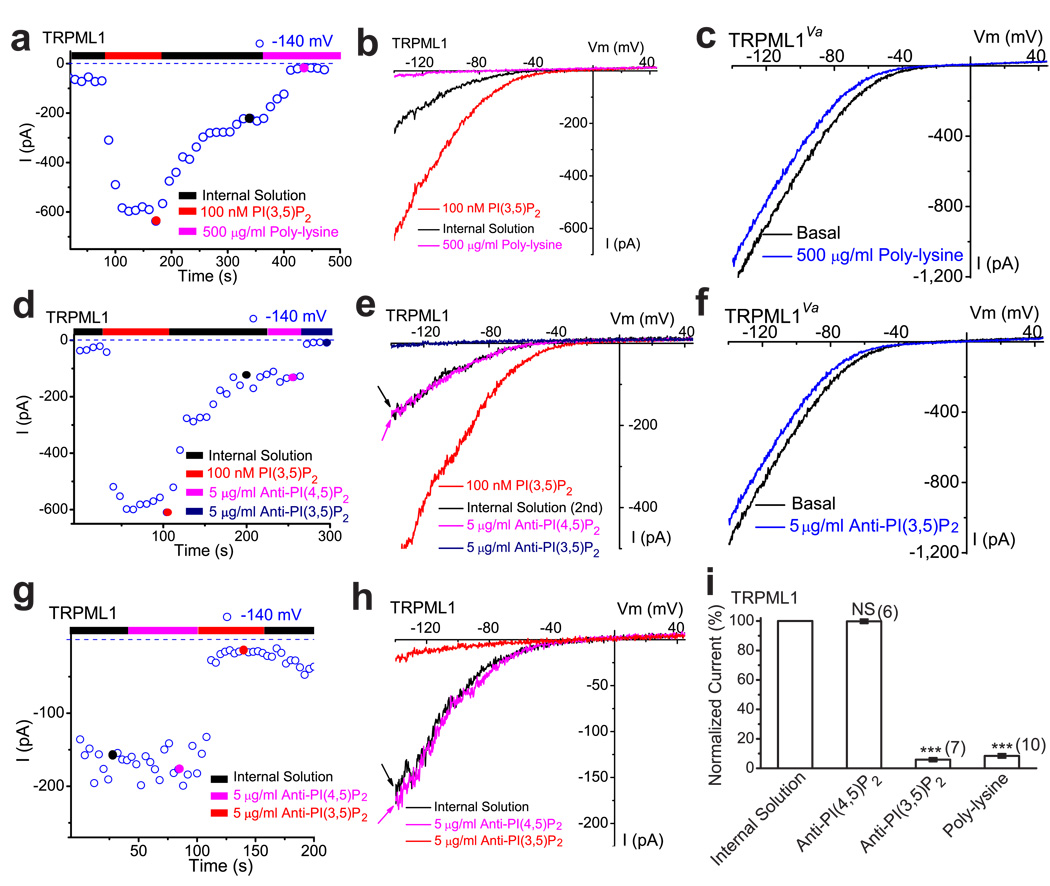
Figure 3. A decrease in PI(3,5)P2 by chelating agents suppresses TRPML1 channel activity in the endolysosomal membrane.
a) Post- PI(3,5)P2 quasi-steady state ITRPML1 was inhibited by bath (internal/cytoplasmic) application of poly-lysine (500 µg/ml) to an enlarged endolysosome from a TRPML1-EGFP-expressing Cos-1 cell. ITRPML1 increased with addition of 100 nM PI(3,5)P2, but gradually reduced to a quasi-steady state level upon washout. b) Representative traces of ITRPML1 at three time points, as shown in a: upon PI(3,5)P2 application (red), washout (black), and poly-lysine application (magenta). c) Lack of poly-lysine (500 µg/ml) effect on whole-endolysosome ITRPML1-Va. d) Post- PI(3,5)P2 quasi-steady state ITRPML1 was inhibited by bath application of neutralizing anti-PI(3,5)P2(5 µg/ml), but not anti-PI(4,5)P2 (5 µg/ml). e) Representative traces of ITRPML1 at three time points, as shown in d. f) Lack of anti- PI(3,5)P2 (5 µg/ml) effect on whole-endolysosome ITRPML1-Va. g, h) Large basal pre- PI(3,5)P2 ITRPML1 was inhibited by bath application of neutralizing anti-PI(3,5)P2 (5 µg/ml), but not anti-PI(4,5)P2. i) ITRPML1 was inhibited by more than 90% by bath (internal/cytoplasmic) application of poly-lysine (500 µg/ml) or PI(3,5)P2 antibody.