Table 2.
Reductive carboxylation substrate scope.
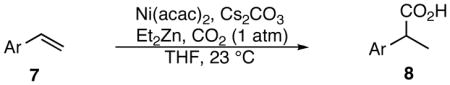 | |||
|---|---|---|---|
| entrya | Aryl Group (Ar) | σm/p/σ+b | yield (%)c |
| 1 |
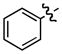 b b
|
0 | 56 |
| 2 |
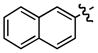 c c
|
- | 60 |
| 3 |
|
- | 66 |
| 4 |
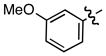 e e
|
0.12 | 92 |
| 5 |
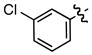 f f
|
0.37 | 68 |
| 6 |
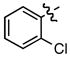 g g
|
- | 65 |
| 7 |
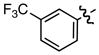 h h
|
0.43 | 79 |
| 8 |
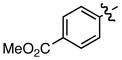 a a
|
0.45/0.48 | 84 |
| 9 |
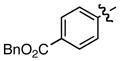 i i
|
0.45/0.48 | 81 |
| 10 |
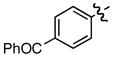 j j
|
0.50/0.51 | 72 |
| 11 |
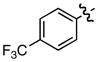 k k
|
0.54/0.61 | 92 |
| 12 |
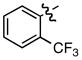 l l
|
- | 87 |
| 13 |
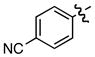 m m
|
0.66/0.66 | 61 |
Standard conditions: Ni(acac)2 (10 mol%), CS2CO3 (20 mol%), Et2Zn (250 mol%), 18 h.
See reference 9.
isolated yield.
