Abstract
This research examines the processes by which patient self-management interventions are related to symptom responses among cancer patients. A total of 333 patients from two randomized clinical trials were combined. Each patient received a six-contact 8-week patient self-management intervention delivered by a nurse to address symptoms. Nurses’ decisions to deliver the strategies, patient enactment of strategies, and their success were investigated using patient- and symptom-level characteristics. Generalized estimating equation modeling accounted for clustering of symptoms and strategies delivered for each symptom within patient. Patient self-management intervention strategies were classified into four classes. Strategies were delivered by nurses for symptoms with higher interference and longer duration. Patient and symptom factors were related to enactment strategies. Symptom responses were related to number of strategies tried by patients. Delivery and enactment of strategies were related to both patient and symptom characteristics.
Keywords: Cancer, Symptom responses, Cognitive behavioral interventions
Introduction
Engaging patients to participate in self-care activities to manage chronic conditions incorporates behavior changes and life style adaptations associated with treatment regimens and their possible side effects [1–5]. Underpinning self-care management strategies is the assumption that effective care must build on collaboration between providers and patients [6, 7]. Models for self-care management have been widely tested and proven effective among patients with such chronic conditions as asthma, diabetes, and hypertension [8, 9]. In a recent publication, the Institute of Medicine highlighted the importance of self-care for persons with cancer [10]. However, there is little information on which components of patient self-management interventions facilitate enactment of patients in self-care management strategies.
This research draws upon cognitive behavioral problem-solving models to examine the processes through which cancer patients engage in self-care management strategies to manage their symptoms during chemotherapy. Patient self-management interventions engage patients in self-care management by allowing them to prioritize problems, offering information, skill building, support and counseling, and prescriptive behaviors that fit within and are tailored to patients’ daily routines [11]. Variations in the number and types of intervention strategies delivered and, in turn, enacted upon, may be associated with patients’ symptom responses. Moreover, patient characteristics, such as age, depressive affect, numbers of comorbid conditions, as well as total symptom burden, and symptom interference with daily activities, may temper how self-care approaches are enacted or are associated with symptom responses [12–15].
Over 325 studies have been published describing outcomes from cognitively based self-care interventions. Several meta-analyses of these trials have been completed as well as a review of meta-analyses [16, 17]. When contrasted with information and educational approaches, these behavioral models appear to be only marginally better [17–20].
Review of the Literature
Cognitive behavioral therapy was introduced by Beck as a set of strategies to address depression [21]. Dobson [22], Persons [23], and McGinn [24] have summarized the common underlying assumptions on which cognitive behavioral approaches rest: (1) recognition that cognition precedes and directs behavior, (2) cognitive behavioral interventions are time limited, (3) clients need to assume control for solving the problems they face, (4) strategies involve isolating problems and teaching clients how to use strategies to solve and to adapt to their situations, and (5) these strategies need to be tailored to the needs of specific clients during the therapeutic process.
Drawing on these precepts, we developed a patient self-management intervention that offered patients information and a series of related strategies focusing on self-care, communication of needs to providers and family, and counseling targeted toward each of the 15 symptoms addressed in this research.
With respect to symptom management, the processes through which patient self-management interventions produce behavior change remain unclear, which strategies are delivered, how well do patients enact those strategies, and do the strategies enacted to lower severity of one symptom have implications for the management of other symptoms [25].
Research into the mechanisms through which patient self-management interventions produce outcomes is evolving. Czaja and colleagues have argued that an intervention should specify the processes leading to a particular outcome and prepare a measurement model that can assess the contributions of each strategy to an outcome [26]. Leventhal and colleagues have argued for a detailed explication of the mechanisms through which these strategies produce the desired outcomes [27].
The following objectives guide this report: (1) to describe the intervention categories and the frequency with which strategies from each category were delivered, the percent of those delivered that were enacted (tried), and the percent of those enacted that were considered helpful by the patients; (2) to identify which symptoms nurse interveners and their patients select for the delivery of strategies; (3) among those strategies delivered, what patient and symptom factors are related to their enactment (trying); and (4) what patient and symptom-related factors, including enactment, produce symptom responses.
Methods
Prior Work Leading to this Analysis
Early findings indicated that when exposed to a patient self-management intervention, cancer patients responded differentially depending upon whether they reported pain, fatigue, or both symptoms [28]. When examined in a randomized trial, a five-contact nurse-delivered intervention proved to lower summed severity scores significantly when compared with conventional care alone. After five contacts over 10 weeks, the nurse arm produced a significant reduction in summed severity compared with the control group. Further, the interaction of trial arm and symptom severity at baseline was significantly favoring the nurse arm over conventional care [29]. Subsequent analyses summarized the moderating effects of depression, neutropenia, and treatment complications on the impact that patient self-management interventions produce with regard to summed symptom severity. In separate models, depression and neutropenia moderated the effects of the experimental arm on symptom severity [30, 31]. While the patient self-management intervention improved symptom management over conventional care, we wanted to determine if the nurse-delivered patient self-management intervention arms were superior to informational approaches in engaging patients in self-care strategies and reducing symptom burden. In two recently completed trials, the nurse-administered arms were compared to active interventions where all patients received the same number of contacts, the same symptoms were assessed, and the only difference was referral of patients to a written symptom management guide for each symptom above threshold. In trial 1, a nurse-delivered patient self-management intervention was compared to a non-nurse coach who assessed symptoms and referred patients to a written symptom management guide. In trial 2, the nurse arm was contrasted with an automated voice response system that queried patients about their symptoms and referred them to the symptom management guide. In both trials, all active arms produced significant reductions in symptom severity over baseline as measured by a summed score of severities across 15 symptoms. In trial 1, the nurse arm reported baseline mean summed severity of 41 (standard deviation 21); the coach arm had a mean of 40 (standard deviation 23). At 10 weeks, scores were 21 (standard deviation 17) and 21 (standard deviation 16) for the nurse and coach arms, respectively. In trial 2 at baseline, the nurse arm reported mean severity of 33 (standard deviation 21) and the automated voice response arm 35 (standard deviation 22). Mean summed severity at 10 weeks was reduced to 20 (standard deviation 19) in the nurse arm and to 20 (standard deviation 18) in the automated voice response arm [20, 32]. While all arms of the two trials produced significant reductions over baseline, no differences between arms of each trial were found. Indirectly, we were curious to learn why these elaborate approaches may have been no better than the information/education arms. Given the significant effects of the nurse arm compared with care as usual, we wanted to deconstruct the nurse-directed patient self-management intervention in order to appreciate how nurses determined which symptoms should receive strategies, which strategies patients tried, and in turn which symptoms responded. Furthermore, results described above were based on a summed symptom severity index. Such a single summary symptom severity burden index has multiple drawbacks [33]. Therefore, a new response analysis methodology was developed and applied to the symptom data collected in the two trials. This analysis revealed which specific symptoms responded by accounting for the associations among multiple symptoms within patient [32–34]. This methodology is applied to the investigation of the patient self-management intervention processes in the present paper.
The nurse arms of the two trials were selected for this process analysis because detailed information about the chosen symptoms and the specific strategies delivered and enacted by patients were only available for the nurse arms. In addition, combining two nurse arms yields the largest sample size for the process analyses. The rationale for combining two arms is that the same nurses were involved in the intervention delivery and both trials shared the same recruitment procedures, both arms had the same nurse protocol for interventions, and both nurse arms produced similar reduction in severity of symptom between intake and 10 weeks.
Sample
The accrual sites for both trials included two comprehensive cancer centers, two community cancer oncology programs, and five hospital-affiliated community oncology centers. The clinical trial offices of these sites assigned nurses, employed by the research study, to implement the recruitment protocol. To be eligible, patients had to meet the following requirements: (1) should be 21 years of age or older; (2) have a diagnosis of a solid tumor or non-Hodgkin lymphoma; (3) should be undergoing a course of chemotherapy at the time of enrollment; (4) should be able to speak and read English, without hearing deficits; and (5) have a touchtone telephone. Both trials were completed between 2004 and 2006.
Patients (and caregivers) who signed human subject consent forms, approved by each site, had their enrollment data entered into a secure website. Prior to trial entry, all patients were screened for severity of 15 symptoms via twice weekly calls for up to 6 weeks. To be eligible for trial 1, patients had to score a severity of 2 or higher on both pain and fatigue on an 11-point scale (0–10) or a three or higher on either pain or fatigue and have a family caregiver who agreed to participate [28]. Patients and their caregivers completed the intake interview, received a copy of the Symptom Management Guide (a manual of strategies for the self-care management of each of the symptoms addressed in the two trials), and were randomized into a six-contact, 8-week trial where they received either the nurse-delivered patient self-management intervention or the coach-delivered information and self-care intervention. In both arms, patients’ symptoms were explained to their caregiver at the first, fourth, and sixth contact. Caregivers were informed to consult the symptom guide to assist their patient with symptom management.
Patients with no caregivers or whose caregivers did not agree to participate were eligible for trial 2. Patients had to report a severity on any of the 15 symptoms of 2 or higher. Patients meeting the criteria, completed a baseline interview, received a Symptom Management Guide, and were then randomized into a six-contact, 8-week trial comparing a nurse-delivered patient self-management intervention with an information and self-care intervention that was delivered via automated voice response system. Only two recruited patients never reached a severity score of 2 or higher on any symptom. They were sent a thank you letter and were not included further. Randomization to arms in both trials was completed using a computer minimization program that balanced arms in each trial with respect to accrual location and site of cancer.
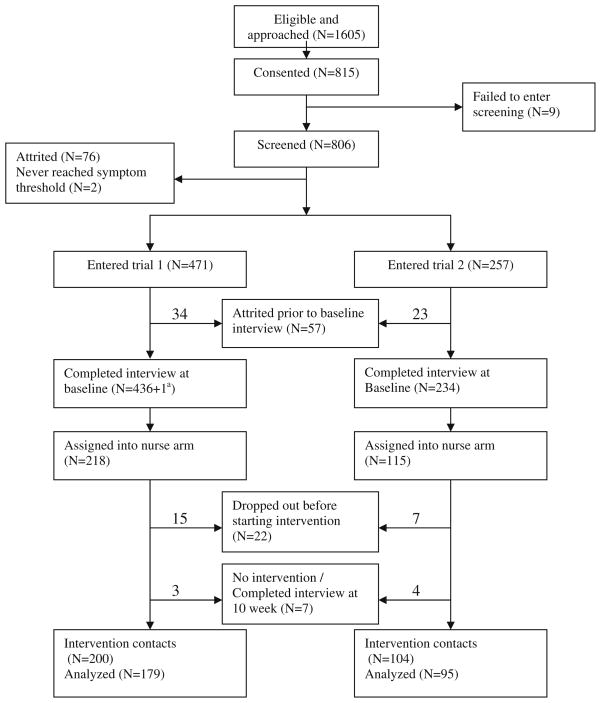
Fig. 1 summarizes the flow of patients from eligibility through randomization, baseline interview, and completion of their intervention contacts. The numbers of patients who skipped the intervention or were lost during the 8-week intervention period are specified by trial.
Fig. 1.
Flowchart of patient accrual and retention for the trials
Measures
Symptom Measures
For these analyses, each of 16 frequently occurring symptoms associated with cancer treatment (anxiety, constipation, cough, depression, diarrhea, dry mouth, difficulty remembering, dyspnea, fatigue, insomnia, nausea and vomiting, pain, peripheral neuropathy, poor appetite, and weakness; alopecia was removed) were assessed at intake, each of the six intervention contacts and at the 10-week endpoint. Three dimensions were identified for each symptom. The severity of each symptom was identified using the same 11-point scale from 0=not present to 10=worst it can be. Patients reporting a severity of 1 or higher were then asked on how many of the past 7 days they experienced the symptom, indicating its duration and using the same 11-point scale. Patients were asked four additional questions regarding how the symptom interfered with their enjoyment of life, relationships with others, general daily activities, and emotions. These four interference items were summed to produce a score ranging from 0 to 40 for each symptom and internal consistency reliability exceeding 0.80. Using a single summed symptom interference score to reflect the reactive dimension of symptom experience has been shown to be valid and reliable [35, 36]. The interference scale is included as a covariate in the models for symptom delivery and enactment, but combined with severity to create response categories.
The severity and interference dimensions of each symptom experience were combined in cut-points for mild, moderate, and severe categories for each symptom established in prior work [35]. Cut-points separating moderate severity from mild and severe from moderate severity scores were based on the largest increases in the interference scale scores between successive increments in symptom severity. Longitudinal analyses based on patients’ reports at each of the six intervention contacts were conducted and showed that these symptom-specific cut-points in severity based upon the magnitude of the interference scale scores consistently differentiated the levels of symptom interference over time (six contacts of the intervention). These analyses are described elsewhere [35, 36].
Based on National Cancer Care Network guidelines [37], symptoms rated at a 4 or higher in severity (on a 0–10-point scale) were eligible to receive interventions under the cognitive behavioral protocol. This pre-established threshold of 4 or higher corresponded to all symptom cut-points as moderate or severe, except for dry mouth, where the moderate category began at 5. The onset for each symptom to receive interventions was defined as the contact number when the symptom first reached a threshold of 4. At the time of onset, symptom severity [4–10], symptom duration (1–7 days) and symptom interference (0 to 40), and the total number of symptoms above threshold were entered into the models to predict nurse delivery and then patient enactment of strategies.
Symptom responses to intervention strategies were defined for each symptom by comparing the onset severity category (severe or moderate) against the severity category at the last contact completed by the patient. Responses were identified as symptom cases moving from severe to moderate or mild and those moving from moderate to mild. Symptom cases remaining severe or moderate or those that transitioned from moderate to severe between onset and the last contact completed by the patient were considered non-responders. Since multiple symptoms were nested within a patient, a patient may be a responder, for example on pain, but a non-responder on fatigue. The symptom cases that remained mild (never reached threshold of 4 or above) were not eligible to receive interventions and were not included in these analyses. Finally, all symptom cases reaching threshold on the last contact were not included in the analysis since there was no opportunity to assess their response.
Patient and Disease Characteristics
Site and stage of cancer were obtained through an audit of patients’ medical records. Other sites of cancer, such as ovarian, uterine, gastrointestinal, head, and neck, were collapsed into a single category because of the small number of cases accrued with these diseases. Patient comorbidity was derived from patients’ reports as described by Katz and colleagues and summarized as a less than three vs. three or more comorbid conditions [38]. The Center for Epidemiologic Studies—Depression (CES-D) scale was administered during the intake interview. Patient responses to 20 CES-D items using a 0–3 rating scale were summed to obtain the total score ranging from 0 to 60 with a Cronbach’s alpha of 0.92 [39]. These variables are summarized in Table 1.
Table 1.
Socio-demographic characteristics of patients by trial
| Trial 1 (N=179)b |
Trial 2 (N=95)c |
||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Patient’s sexa | Male | 44 | 24.58 | 40 | 42.11 |
| Female | 135 | 75.42 | 55 | 57.89 | |
| Cancer sitea | Breast | 72 | 40.68 | 25 | 26.32 |
| Lung | 29 | 16.38 | 30 | 31.58 | |
| Colon | 29 | 16.38 | 9 | 9.47 | |
| Other | 47 | 26.55 | 31 | 32.63 | |
| Cancer stage | Early | 46 | 25.99 | 15 | 15.79 |
| Late | 131 | 74.01 | 80 | 84.21 | |
| CES-D | Less than 16 | 123 | 68.72 | 67 | 70.53 |
| 16+ | 56 | 31.28 | 28 | 29.47 | |
| Comorbidity | Less than 3 | 111 | 62.01 | 61 | 64.21 |
| 3+ | 68 | 37.99 | 34 | 35.79 | |
Chi-square; p value significant under the criteria p<0.05
Patient’s age in years (standard deviation (SD))=56.89 (11.79)
Patient’s age in years (SD)=57.65 (10.68)
Table 2 describes for each symptom the mean severity at onset, duration and interference scores as well as the burden of other symptoms present at onset, and the number of patients with each symptom where no strategies were delivered, the mean number of contacts each symptom was open, and the number and percent of patients reporting each symptom who responded.
Table 2.
Number of patients who reported symptom over threshold, mean of severity, duration, interference at onset, total number of symptoms above threshold at onset, and response rate for each symptom
| Symptom | N of patient onset | Severity onset, mean (SD) | Duration onset, mean (SD) | Interference onset, mean (SD) | Number of symptoms over threshold at onset, mean (SD) | Number of patients with no interventions delivered, N (%a) | Number of contact over threshold, mean (SD) | Response, N (%a) |
|---|---|---|---|---|---|---|---|---|
| Anxiety | 120 | 5.4 (1.4) | 4.3 (2.2) | 14.6 (8.9) | 5.0 (2.7) | 21 (17.5) | 2.1 (1.2) | 95 (79.2) |
| Constipation | 72 | 5.9 (1.9) | 3.8 (1.9) | 8.7 (9.0) | 4.9 (2.5) | 10 (13.9) | 2.0 (1.2) | 56 (77.8) |
| Cough | 63 | 5.7 (1.7) | 5.5 (1.9) | 9.1 (8.6) | 5.9 (2.6) | 24 (38.1) | 1.9 (1.4) | 50 (79.4) |
| Depression | 82 | 5.4 (1.5) | 4.5 (2.0) | 17.0 (9.1) | 6.1 (2.5) | 18 (22.0) | 1.9 (1.2) | 60 (73.17) |
| Diarrhea | 53 | 6.1 (2.1) | 3.1 (2.2) | 12.3 (11.3) | 5.3 (2.8) | 13 (24.5) | 1.7 (1.1) | 45 (84.9) |
| Difficulty remembering | 81 | 5.2 (1.4) | 6.0 (1.5) | 8.1 (8.1) | 5.5 (2.6) | 35 (43.2) | 2.0 (1.2) | 53 (65.4) |
| Dry mouth | 82 | 6.4 (1.5) | 6.0 (1.8) | 4.5 (6.3) | 5.0 (2.3) | 18 (22.0) | 2.2 (1.3) | 68 (82.9) |
| Dyspnea | 73 | 5.4 (1.5) | 5.1 (2.1) | 13.6 (10.0) | 5.3 (2.9) | 15 (20.6) | 2.2 (1.3) | 39 (53.4) |
| Fatigue | 213 | 5.7 (1.7) | 5.3 (2.0) | 13.7 (9.6) | 4.4 (2.5) | 7 (3.3) | 2.8 (1.6) | 112 (52.6) |
| Insomnia | 132 | 5.9 (1.6) | 5.2 (2.1) | 9.3 (8.6) | 4.7 (2.7) | 29 (22.0) | 2.3 (1.4) | 102 (77.3) |
| Nausea/vomiting | 69 | 5.9 (1.9) | 3.7 (2.3) | 14.5 (10.8) | 5.4 (2.6) | 16 (23.2) | 1.8 (1.2) | 59 (85.5) |
| Pain | 111 | 5.6 (1.8) | 4.9 (2.0) | 14.0 (11.2) | 5.0 (2.5) | 9 (8.1) | 2.2 (1.4) | 76 (68.5) |
| Peripheral neuropathy | 77 | 5.6 (1.7) | 5.9 (1.8) | 7.0 (7.6) | 4.8 (2.8) | 15 (19.5) | 3.0 (1.6) | 40 (52.0) |
| Poor appetite | 133 | 5.7 (1.8) | 5.0 (2.1) | 8.6 (8.4) | 4.9 (2.6) | 23 (17.3) | 2.1 (1.2) | 103 (77.4) |
| Weakness | 129 | 5.5 (1.6) | 5.8 (1.8) | 11.7 (9.5) | 5.1 (2.5) | 40 (31.0) | 2.3 (1.4) | 93 (72.1) |
Percentage based on number of patients with symptom severity over threshold at onset
The Intervention
The nurse-administered self-management intervention was implemented as an arm in both trials 1 and 2. The same nurses used identical software to assess and rate symptoms, to select and record intervention strategies delivered, and catalog patients’ reports of the strategies tried. The protocol restricted nurses from intervening on more than four symptoms per contact and from delivering more than four strategies per symptom. In collaboration with their patients, nurses prioritized which symptoms to address and selected from drop-down menus from their computer’s intervention approaches for engaging patients in the management of their symptoms. Strategies were classified according to four themes: self-care behaviors (adherence to medications, diet, exercise), information and decision-making (cuing strategies, prioritizing, and limiting daily tasks), communication with family and providers (report problems, engage help, script out questions for providers), and counseling and support (coping strategies, re-framing) [40–43].
Once selected, the nurse reviewed each strategy with the patients and helped them to plan for implementation. At subsequent contacts, nurses began the session by asking patients if they had tried (enacted) each strategy recommended at the previous contact and, if so, asked them to identify how helpful each strategy was for managing the symptom. Interventions, not tried or unsuccessful, could be replaced with new ones. Successful interventions could be continued until the symptom was managed. Table 3 summarizes the number of patients and symptom cases reporting each symptom over threshold, total number of strategies delivered, and mean number of strategies delivered per patient per symptom. The final columns describe for each approach (self-care, information, communication, and counseling) the number of strategies delivered enacted and reported as helpful by patients.
Table 3.
Strategies delivered for each symptom by intervention category
| Symptom |
N of casesa |
Deliveredb | Average deliveredc |
Self-care |
Information |
Counseling |
Communication |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delivered,d N (%) |
Enact,eN (%) | Helped,f N (%) |
Delivered,d N (%) |
Enact,e N (%) |
Helped,fN (%) | Delivered,d N (%) |
Enact,e N (%) |
Helped,f N (%) |
Delivered,d N (%) |
Enact,e N (%) |
Helped,f N (%) |
||||
| Anxiety | 247 | 785 | 3.18 | 360 (46) | 251 (70) | 243 (97) | 171 (22) | 136 (80) | 124 (91) | 158 (20) | 144 (91) | 136(94) | 96 (12) | 47 (49) | 43(91) |
| Constipation | 146 | 447 | 3.06 | 249 (56) | 202 (81) | 179 (89) | 136 (30) | 121 (89) | 104(86) | 26 (6) | 24 (92) | 23 (96) | 36 (8) | 18 (50) | 17 (94) |
| Cough | 122 | 261 | 2.14 | 134 (51) | 89 (66) | 86 (97) | 75 (29) | 55 (73) | 52 (95) | 14 (5) | 14 (100) | 14 (100) | 38 (15) | 18 (47) | 18 (100) |
| Depression | 156 | 437 | 2.80 | 192 (44) | 130 (68) | 127 (98) | 106 (24) | 86 (81) | 76 (88) | 73 (17) | 49 (67) | 48 (98) | 66 (15) | 40 (61) | 37 (93) |
| Diarrhea | 92 | 245 | 2.66 | 119 (49) | 89 (75) | 80 (90) | 86 (35) | 74 (86) | 62 (84) | 15 (6) | 14 (93) | 13 (93) | 25 (10) | 12 (48) | 11 (92) |
| Difficulty remembering | 162 | 259 | 1.60 | 171 (66) | 120 (70) | 110 (92) | 50 (19) | 38 (76) | 34 (89) | 26 (10) | 23 (88) | 21 (91) | 12 (5) | 6 (50) | 6 (100) |
| Dry mouth | 178 | 425 | 2.39 | 246 (58) | 176 (72) | 174 (99) | 145 (34) | 119 (82) | 96 (81) | 24 (6) | 21 (88) | 21 (100) | 10 (2) | 4 (40) | 3 (75) |
| Dyspnea | 160 | 510 | 3.19 | 272 (53) | 174 (64) | 164 (94) | 134 (26) | 96 (72) | 75 (78) | 39 (8) | 34 (87) | 34 (100) | 65 (13) | 30 (46) | 28 (93) |
| Fatigue | 588 | 2299 | 3.91 | 1326 (58) | 1057(80) | 988 (93) | 609 (26) | 530(87) | 478 (90) | 235 (10) | 221(94) | 219 (99) | 129 (6) | 75 (58) | 61 (81) |
| Insomnia | 305 | 790 | 2.59 | 401 (51) | 258 (64) | 228 (88) | 231 (29) | 181 (78) | 149 (82) | 77 (10) | 74 (96) | 71 (96) | 81 (10) | 41 (51) | 34 (83) |
| Nausea/vomiting | 121 | 339 | 2.80 | 166 (49) | 124 (75) | 108 (87) | 117 (35) | 98 (84) | 86 (88) | 18 (5) | 18 (100) | 18 (100) | 38 (11) | 21 (55) | 18 (86) |
| Pain | 241 | 854 | 3.54 | 375 (44) | 234 (62) | 223 (95) | 283 (33) | 229 (81) | 186 (81) | 60 (7) | 53 (88) | 51 (96) | 136 (16) | 79 (58) | 72 (91) |
| Peripheral neuropathy | 228 | 507 | 2.22 | 272 (54) | 200 (74) | 182 (91) | 148 (29) | 123 (83) | 107 (87) | 45 (9) | 45 (100) | 43 (96) | 42 (8) | 23 (55) | 21 (91) |
| Poor appetite | 285 | 889 | 3.12 | 467 (53) | 318 (68) | 298 (94) | 293 (33) | 242 (83) | 217 (90) | 82 (9) | 75 (91) | 73 (97) | 47 (5) | 23 (49) | 21 (91) |
| Weakness | 298 | 571 | 1.92 | 300 (53) | 206 (69) | 178 (86) | 182 (32) | 153 (84) | 134 (88) | 54 (9) | 48 (89) | 46 (96) | 35 (6) | 21 (60) | 21(100) |
Number of cases with severity over 4. For example, during intervention, a patient reported pain three times over threshold; the number of cases with severity over 4 for pain of this patient would be 3
Total number of strategies being delivered during intervention for that symptom
The average delivered is the average number of strategies being delivered for each symptom case
Number of each type of strategy being delivered during intervention. The percentage is for each type of strategy by total number of strategies being delivered
Number of each type of strategy being enacted. The percentage is for each type of strategy being enacted by number of strategies being delivered
Number of each type of strategy reported by patient as helped. The percentage is for each type of strategy patient reported as helped by the number of strategies patient enacted
Data Analysis
The two nurse arms from the two trials were compared at baseline. The variables with respect to which of the two arms differed as well as the trial variable itself were adjusted for in the analyses described below. The first set of analyses answered the question about nurses’ and patients’ selections of which symptoms to intervene on and which symptoms to defer from intervening on. Because strategies were symptom-specific, available data were at the symptom-case level. These symptom-case data were aggregated within patient using generalized estimating equations (GEE) technique for the models where the outcome was binary [44] and using mixed effects models where the outcome was treated as approximately continuous. Binary outcome variables were delivery of strategy for a symptom (yes/no any time during the contacts for symptoms that reached threshold of 4), and symptom response. Mixed modeling was implemented for the number of enacted strategies, which were in an approximately normal distribution. In both GEE and mixed models, symptom cases were considered nested within patients, and compound symmetry association structure among symptoms within patient was specified. Symptom-specific intercepts represented differences among symptoms and reflected the fact that all other factors being equal, a strategy may be more likely to be delivered or enacted, or response may be more likely to occur for one symptom, than another. In addition, our analytic strategy allowed for inclusion of both symptom-level variables and patient-level variables to explain variation in the delivery and enactment of strategies and symptom response. Thus, the explanatory variables were symptom severity, symptom duration, interference at onset, total number of symptoms above threshold at onset, the number of comorbid conditions, depressive symptomatology, age, sex, site, and stage of cancer. For the outcome of the number of strategies tried, symptom-specific intervention delivery variable was added as a covariate, and only those symptom cases where strategies were delivered were included. In the response analysis, symptom interference at onset was removed from the list of covariates because symptom interference was implicitly included in the response outcome variable. Because responses to different symptoms may be associated regardless of delivery of the intervention, the analysis of symptom response included symptom cases that received the interventions as well as those that did not. In order to do that, the number of interventions tried for each symptom was dichotomized at the median as 5 or more vs. less than 5, and one more level, “no interventions delivered,” was added. Finally, in addition to formal statistical analysis of response data, response rates were presented in graphic form according to the following: tried fewer than five strategies, tried five or more strategies, and no strategies delivered.
Results
Table 1 summarizes patients’ socio-demographic characteristics including age, sex, sites of cancer, stage, baseline CES-D depression scores, and comorbidity, for the cognitive behavioral arms in trials 1 and 2. Chi-square tests revealed that trial 1 had more female patients and, thus, different distribution of site of cancer compared to trial 2. No other differences between the nurse arms of two trials were found at baseline. At intake, all patients in both trials were undergoing chemotherapy. We compared those patients whose chemotherapy ended prior to the end of the intervention with patients whose treatment extended beyond. Two hundred and five patients continued treatment beyond the end of the trials and 69 completed treatment before the end of the trials. Mean severity at 10 weeks adjusted for baseline severity indicated no significant difference between those still on chemotherapy and those who completed chemotherapy (data not shown).
Table 2 summarizes the symptom-level variables including onset severity; duration and interference; the number of other symptoms that were over threshold at the time of onset of each symptom; and the mean number of contacts over threshold. The final column contains the response rates for each symptom that ranged from 52% for peripheral neuropathy to 85% for diarrhea.
Table 3 describes the number of cases reporting each symptom above threshold, the total number strategies delivered, and the average strategies delivered for each symptom, each time that symptom was reported above threshold. The second part of Table 3 separates the total number of strategies delivered, enacted (tried), and reported as helpful, according to the four categories from which strategies were delivered by nurses, enacted by patients, and patients perceived helpfulness of that category of strategies for managing that symptom. Self-care behavioral strategies accounted for slightly over half of all interventions delivered. Information approaches accounted for 25–30% with counseling followed by communication approaches completing the frequency with which strategies were delivered by category. Patients were more likely to have self-care and information approaches delivered and less likely to have counseling and communication approaches delivered. Self-care, information, and counseling approaches—each depending on patient actions—all had higher rates of enactment than communication that required interactions with family or health professionals. Finally, when patients enacted strategies, they almost always rated them as helpful. The high rates of helpfulness among strategies enacted suggests that patients either engaged in only those strategies they thought would benefit them, or high level of helpfulness indicates an unwillingness of patients to acknowledge that they tried strategies that were not beneficial.
In Appendix 1, the five most frequent interventions from each category (self-care, information, communication, and counseling) are presented for fatigue. Fatigue was selected because it was the most prevalent symptom, took the most contact to achieve responses, and had among the lowest rates of response. As a result, fatigue had the most interventions delivered from each of the categories. Self-care strategies were most often delivered for fatigue and the five most frequent interventions accounted for 60% (801/1,326) of all self-care interventions for fatigue. For information, the five most frequent interventions accounted for 42% (255/609), and for communication and counseling, the top five interventions accounted for 88% (113/129) and 69% (162/235), respectively. For self-care and information, many of the strategies were virtually identical but were contextualized by the nurses. Fatigue management, exercise, diet, and nutrition appear in both self-care and information categories. Nurses began by describing exercise, diet, hydration, and nutrition, and subsequently for patients who questioned the strategies or were unable to try them or failed to see them as helpful; the nurses would use the information category to inform patients as to why and how each of these activities could help to lower their fatigue. This example, using fatigue, reflects the order in which nurses selected specific interventions from the four categories for the other symptoms. The specific interventions nested within each category were more similar than different among symptoms. Finally, the percent of the five most frequently occurring interventions enacted and deemed helpful by patients reflects the percents for their corresponding categories in Table 3.
Appendix 1.
Five Most Frequent Strategies by Category, for Fatigue
| Self Care | |||||
|---|---|---|---|---|---|
| Strategy | Number Delivered | Number Tried | Percent Tried | Number Helped | Percent Helped |
| Fatigue management (exercise, nutrition, fluids) | 228 | 213 | 93.4 | 206 | 96.7 |
| Toolkit use | 216 | 91 | 42.1 | 88 | 96.7 |
| Fluids/hydration (8 glasses per day) | 186 | 169 | 90.9 | 146 | 86.4 |
| Diet/nutrition | 88 | 76 | 86.4 | 64 | 84.2 |
| Energy conservation (balance sleep, exercise/activity) | 83 | 71 | 85.5 | 68 | 95.8 |
| Information | |||||
|---|---|---|---|---|---|
| Strategy | Number Delivered | Number Tried | Percent Tried | Number Helped | Percent Helped |
| Fatigue management (exercise, nutrition, fluids) | 62 | 61 | 98.4 | 59 | 96.7 |
| Toolkit use | 53 | 36 | 67.9 | 25 | 69.4 |
| Fluids/hydration (8 glasses per day) | 48 | 45 | 93.8 | 41 | 91.1 |
| Diet/nutrition | 46 | 42 | 91.3 | 40 | 95.2 |
| Exercise everyday | 46 | 39 | 84.8 | 35 | 89.7 |
| Communication | |||||
|---|---|---|---|---|---|
| Strategy | Number Delivered | Number Tried | Percent Tried | Number Helped | Percent Helped |
| Communicate in general about symptom | 32 | 15 | 46.9 | 10 | 66.7 |
| Amount and patterns of fatigue/overwhelmed | 25 | 17 | 68.0 | 11 | 64.7 |
| Unable to do usual activities/interference with function/unable to move for 24 hours | 23 | 9 | 39.1 | 8 | 88.9 |
| Need assistance with household chores | 20 | 15 | 75.0 | 15 | 100 |
| How fatigue affects daily activity/emotions | 13 | 11 | 84.6 | 9 | 81.8 |
| Counseling | |||||
|---|---|---|---|---|---|
| Strategy | Number Delivered | Number Tried | Percent Tried | Number Helped | Percent Helped |
| Active listening/guidance/anticipatory guidance | 69 | 67 | 97.1 | 67 | 100 |
| Lifestyle changes | 32 | 30 | 93.8 | 30 | 100 |
| Problem solving | 28 | 27 | 96.4 | 27 | 100 |
| Optimistic thinking | 18 | 17 | 94.4 | 17 | 100 |
| Family support | 15 | 11 | 73.3 | 11 | 100 |
Delivery of Strategies
Step 1 in the analyses was to determine what patient characteristics and which symptoms, compared to weakness as a reference, predicted delivery of intervention. Strategies were delivered for symptoms with higher severity at onset, a longer duration over threshold during the past 7 days, and for those with greater interference (see Table 4). The total number of symptoms above threshold at the time of symptom onset was negatively associated with delivery. This finding was related to the fact that nurses were constrained by the protocol to deliver strategies for no more than four symptoms. Site, other than lung cancer (an outcome predictor in previous work [20]) was significantly related to delivery, but depressive symptoms and number of comorbid conditions, both observed at baseline, were unrelated to delivery. Delivery of strategies differed by symptom. When compared against weakness (selected alphabetically as the reference symptom), pain and fatigue have the highest probability of having strategies delivered, followed by constipation, depression, poor appetite, dyspnea, anxiety, diarrhea, and other symptoms. Finally, when tested, patient age and sex were not significant predictors in the models, did not change the effects of other variables, and therefore were removed. Trial arm was not significant in this or other statistical models; however, it was retained in all models to control for the fact that data from the two studies were combined.
Table 4.
Adjusted odds ratios (OR) of nurses’ decision to deliver interventions derived from GEE aggregate symptom model
| Adjusted OR (95% CI) | p value | ||
|---|---|---|---|
| Trial (ATSM vs. P&F) | 1.15 (0.78, 1.70) | 0.4820 | |
| Symptom severity at onseta | 1.13 (1.02, 1.26) | 0.0174 | |
| Symptom duration at onseta | 1.10 (1.02, 1.18) | 0.0171 | |
| Interference at onseta | 1.03 (1.00, 1.05) | 0.0306 | |
| Total number of symptoms over thresholda | 0.64 (0.58, 0.72) | <0.0001 | |
| CES-D (16+ vs. less than 16)b | 0.88 (0.56, 1.37) | 0.5642 | |
| Comorbidity (3+ vs. less than 3)b | 0.85 (0.58, 1.24) | 0.3883 | |
| Number of contact over thresholda | 2.07 (1.75, 2.46) | <0.0001 | |
| Cancer stage (early vs. late)b | 1.65 (0.96, 2.84) | 0.0684 | |
| Cancer site (not lung vs. lung)b | 1.67 (1.09, 2.57) | 0.0192 | |
| Symptom (vs. weakness) | Anxietyc | 3.29 (1.73, 6.24) | <0.0001d |
| Constipationc | 5.28 (2.24, 12.44) | ||
| Cough | 1.58 (0.71, 3.51) | ||
| Depressionc | 3.63 (1.67, 7.86) | ||
| Diarrheac | 3.06 (1.28, 7.31) | ||
| Difficulty remembering | 0.82 (0.42, 1.59) | ||
| Dry mouth | 1.99 (0.99, 4.00) | ||
| Dyspneac | 3.39 (1.43, 8.02) | ||
| Fatiguec | 11.35 (5.08, 25.33) | ||
| Insomnia | 1.80 (0.97, 3.33) | ||
| Nausea/vomitingc | 2.94 (1.38, 6.24) | ||
| Painc | 9.57 (3.65, 25.13) | ||
| Peripheral neuropathy | 1.95 (0.78, 4.89) | ||
| Poor appetitec | 3.49 (1.97, 6.18) |
Covariates for symptom-specific
Covariates for patient-specific
p<0.05 for comparison to symptom of weakness
Overall test of any symptom differences on interventions delivered
Enactment of Strategies
Step 2 in the analyses sought to determine the patient characteristics and to identify those symptoms related to the strategies that patients tried. After adjusting for the number of strategies delivered, the higher the symptom interference scores, and the number of contacts where a symptom remained above threshold, the more strategies patients were likely to try (Table 5). However, the number of symptoms over threshold was negatively associated with numbers of strategies that patients tried. Finally, non-lung cancer patients tried more strategies than patients with lung cancer. Overall when compared against weakness, patients tried significantly more strategies for fatigue and constipation (with p value as <0.01 and 0.03 not listed in the table) and marginally significantly fewer for insomnia (p value 0.06, not listed in the table).
Table 5.
Number of interventions tried after adjusting for number of strategies delivered and other patient and symptom covariates
| Estimated Parameter (SD error) | p value | ||
|---|---|---|---|
| Trial (ATSM vs. P&F) | −0.096 (0.157) | 0.5390 | |
| Symptom severity at onseta | −0.025 (0.032) | 0.4329 | |
| Symptom duration at onseta | 0.024 (0.026) | 0.3405 | |
| Interference at onseta | 0.014 (0.007) | 0.0354 | |
| Total number of symptoms over thresholda | −0.102 (0.030) | 0.0008 | |
| CES-D (16+ vs. less than 16)b | −0.097 (0.172) | 0.5739 | |
| Comorbidity (3+ vs. less than 3)b | 0.198 (0.158) | 0.2104 | |
| Number of contact over thresholda | 0.123 (0.048) | 0.0111 | |
| Cancer stage (early vs. late)b | 0.347 (0.180) | 0.0548 | |
| Cancer site (not lung vs. lung)b | 0.394 (0.190) | 0.0392 | |
| Number of strategies being delivereda | 0.735 (0.013) | <0.0001 | |
| Symptom (vs. weakness) | Anxiety | −0.007 (0.234) | <0.0001d |
| Constipationc | 0.602 (0.264) | ||
| Cough | −0.176 (0.309) | ||
| Depression | −0.139 (0.262) | ||
| Diarrhea | 0.108 (0.304) | ||
| Difficulty remembering | −0.214 (0.284) | ||
| Dry mouth | 0.216 (0.265) | ||
| Dyspnea | −0.418 (0.272) | ||
| Fatiguec | 0.748 (0.204) | ||
| Insomnia | −0.434 (0.230) | ||
| Nausea/vomiting | 0.183 (0.279) | ||
| Pain | −0.238 (0.231) | ||
| Peripheral neuropathy | 0.128 (0.265) | ||
| Poor appetite | 0.008 (0.227) |
Covariates for symptom-specific
Covariates for patient-specific
p<0.05 for comparison to symptom of weakness
Overall test of any symptom differences on interventions tried
Symptom Responses
The third step in the analyses examined patient and symptoms associated with higher rates of response. Patients trying five or more strategies, vs. those trying fewer than five, were associated with higher probabilities of response as reported in Table 6 and shown in Fig. 2. Compared with symptoms that received fewer than five strategies, those receiving no strategies were significantly more likely to respond by the end of the study. From this, it seems reasonable to conclude that nurses, together with patients, identified correctly those symptoms that could benefit from management strategies. Symptom severity at onset was significantly related to proportion of symptoms that responded. This may be due to regression to the mean, the patterns in symptom severity over time [45], or an artifact of the scoring procedure where severe symptoms could achieve a response by moving from severe to either moderate or a mild category. Even though the level of depressive symptoms at baseline did not influence the number of interventions tried, patients scoring a 16 or higher on the CES-D were less likely to respond to those symptoms where strategies were tried. At the symptom-case level, compared with weakness (the referent symptom), only fatigue and dyspnea were significantly less likely to respond.
Table 6.
Aggregate effect of GEE model on interventions tried on symptom responses after adjusting for patient and symptom characteristics
| Adjusted OR (95% CI) | p value | ||
|---|---|---|---|
| Trial (ATSM vs. P&F) | 0.79 (0.54, 1.16) | 0.2222 | |
| Symptom severity at onseta | 1.55 (1.38, 1.74) | <0.0001 | |
| Symptom duration at onseta | 0.98 (0.91, 1.05) | 0.5139 | |
| Total number of symptoms over thresholda | 1.00 (0.93, 1.08) | 0.9936 | |
| CES-D (16+ vs. less than 16)b | 0.65 (0.43, 0.98) | 0.0379 | |
| Comorbidity (3+ vs. less than 3)b | 0.87 (0.60, 1.27) | 0.4792 | |
| Number of contacts over thresholda | 0.40 (0.34, 0.46) | <0.0001 | |
| Cancer stage (early vs. late)b | 1.51 (0.94, 2.43) | 0.0876 | |
| Cancer site (not lung vs. lung)b | 1.12 (0.73, 1.70) | 0.6069 | |
| Compliance (tried 5+ vs. less than 5)a | 1.72 (1.20, 2.49) | 0.0036 | |
| (Not delivered vs. less than 5) | 1.56 (1.05, 2.31) | 0.0265 | |
| Symptom (vs. weakness) | Anxiety | 1.20 (0.63, 2.29) | <0.0001d |
| Constipation | 0.92 (0.45, 1.89) | ||
| Cough | 1.29 (0.52, 3.16) | ||
| Depression | 0.76 (0.40, 1.41) | ||
| Diarrhea | 1.12 (0.48, 2.62) | ||
| Difficulty remembering | 0.56 (0.30, 1.04) | ||
| Dry mouth | 1.94 (0.75, 4.98) | ||
| Dyspneac | 0.34 (0.16, 0.71) | ||
| Fatiguec | 0.39 (0.22, 0.67) | ||
| Insomnia | 1.07 (0.56, 2.02) | ||
| Nausea/vomiting | 1.30 (0.57, 2.96) | ||
| Pain | 0.65 (0.36, 1.18) | ||
| Peripheral neuropathy | 0.50 (0.25, 1.03) | ||
| Poor appetite | 1.03 (0.57, 1.88) |
Covariates for symptom-specific
Covariates for patient-specific
p<0.05 for comparison to symptom of weakness
Overall test of any symptom differences on symptom responses
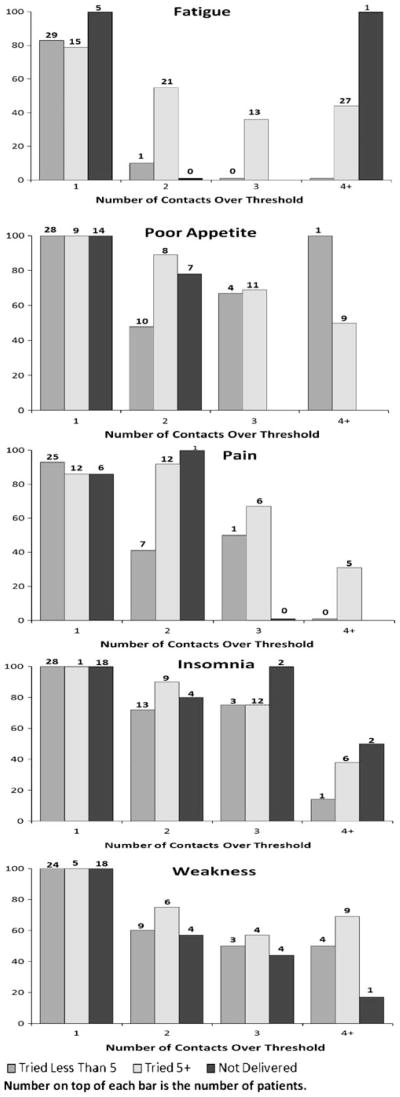
Fig. 2.
Proportion of responses by number of contacts over threshold for five prevalent symptoms
Fig. 2 presents in graphic form the relationships among the number of strategies tried (five or more, less than five, and no strategies delivered) across successive contacts (1–4+) and the resulting proportion of responses for five frequently occurring symptoms. The graphs for these five prevalent symptoms illustrate the increasing strength of the association between numbers of strategies tried and responses for patients where these symptoms persist over successive contacts. Among patients who report fatigue with only one contact and who had no strategies delivered, all five respond spontaneously. Patients with symptoms above threshold at only one contact and who tried fewer than five, or five or more strategies, achieve similar rates of response. This pattern persists across all other symptoms above threshold at a single contact (poor appetite, pain, insomnia, and weakness). However, as symptoms persist above threshold over two, three, and four plus contacts, patients who tried five or more strategies are more likely to achieve responses than those who attempt fewer than five strategies.
Discussion
This research sought to describe the processes through which self-management approaches led to improving cancer patients’ capacities to manage their symptoms. This research examined the impact of both symptom and patient characteristics on the strategies that were delivered and enacted upon symptom responses. Given the disproportionate number of self-care strategies delivered, it was not possible to introduce the four approaches into the models.
Different attributes of symptoms predicted the delivery and enactment of strategies as well as patients’ responses. The duration of a symptom and its interference were associated with patients and nurses decisions to deliver strategies. Interference was related to patients enacting strategies delivered to them. The number of contacts where a symptom remained above threshold was a significant predictor of the decision for patient enactment and subsequently symptom response. Thus, the persistence of symptoms prompts nurses to deliver interventions and patients to try those strategies, which in turn is related to symptom responses.
Even though a limit was set by protocol for the number of symptoms where strategies could be delivered, patients with higher numbers of symptoms were less likely to enact strategies delivered to them. This suggests that patient self-management interventions may be less effective as patients with higher symptom burden attempt to process and then allocate time and effort to focus on multiple strategies requiring differing skills and supports.
At the patient level, those with more depressive symptoms at baseline were less likely to respond, even though they enacted strategies at rates comparable to those with fewer depressive symptoms. Whether this is due to the possibility that more patients with depressive symptoms have greater interference or that the patient self-management intervention strategies were perceived to be ineffective by patients remains an open question. In general, the findings indicate that all patients who try more strategies do receive more responses, but the rate of the responses varies by symptom.
Finally, among patients whose symptoms persist over successive contacts trying five or more strategies result in significantly greater likelihood of achieving a response. Having no interventions delivered results in spontaneous responses mainly for symptoms above threshold at one contact, but as the number of contacts over threshold increases, the proportion of responses where no strategies were delivered declines. Among symptoms where patients tried fewer than five strategies, their pattern of responses approximates those where no strategies were delivered. Patients trying five or more strategies for symptoms above threshold at one contact above threshold do equally as well as those with fewer than five or who receive no strategies to manage their symptom. However, as the number of contacts increase, then patients trying five or more strategies for symptoms above threshold achieve a substantially greater response than those who tried fewer than five strategies. This finding suggests that patient self-management intervention approaches to symptom management require some persistence on the part of patients to achieve a response to their symptoms.
Considering the costs of implementing patient self-management interventions, it is critical to better understand the conditions and circumstances around which they produce symptom responses. Finally, more work is needed to document factors affecting patient enactment of the strategies that are delivered.
Deconstructing the processes and mechanisms of multiple contact, patient self-management interventions on symptom management are complex but necessary to advance this science. First, with respect to symptoms, we demonstrated that attributes such as duration, interference, and persistence are related differently to delivery, enactment, and resulting responses. Second, patient characteristics were examined as well. Together, these patient and symptom-case variables elaborated the processes by which patient self-management interventions can influence symptom outcomes. This effort built upon the work by Czaja [26], Leventhal [27], and Bellg [46]. These lines of investigation point to the complexity inherent in behavioral and self-management trials; further elaboration and replication will be necessary to link intervention dose with patient enactment that leads to specific desired therapeutic outcomes.
Limitations
Several shortcomings of this work deserve consideration. First, we were unable to assess the unique impact of the patient self-management intervention categories. Second, while we observed no differences between the nurse intervention arms, possible variations could be involved in the delivery, enactment, or responses. The number of strategies tried was significant; however, we do not know if there was a sentinel strategy that, when tried, produced a response. Alternative strategies, such as pharmaceutical interventions, could have explained some responses. We report on patients’ indication of the helpfulness of a strategy which might mean it kept that symptom from getting worse and may not be related to a response. Further, we could not identify the “transference” of strategies delivered or enacted for one symptom, which patients may have applied to the management of other symptoms. Despite these limitations, this work does suggest how, and under what conditions, delivery and the enactment of strategies play in achieving responses.
Conclusions
This research examined the processes through which patient self-management models address the management of disease- and treatment-related symptoms among cancer patients undergoing chemotherapy. Symptom-specific variables were associated with nurses’ decisions to deliver symptom management strategies for specific symptoms, and both symptom-specific and patient-specific variables were related with symptom responses. In general, nurses selected symptoms and delivered interventions based on their severity at each contact. This work points to the need for more research regarding the conditions under which symptoms that receive interventions either respond or fail to do so and to determine what factors contribute to the responses of symptoms that do not receive interventions.
Acknowledgments
Acknowledgement of Research Support National Cancer Institute Grant #RO1 CA30724
Automated Telephone Monitoring for Symptom Management, Charles Given, PI, Barbara Given, Co-PI
In Affiliation with the Walther Cancer Institute Indianapolis, IN
Contributor Information
Charles W. Given, Email: givenc@msu.edu, Department of Family Medicine, Michigan State University, East Lansing, MI, USA. B108 Clinical Center, East Lansing, MI 48824, USA
Barbara A. Given, College of Nursing, Michigan State University, East Lansing, MI, USA
Alla Sikorskii, Department of Statistics and Probability, Michigan State University, East Lansing, MI, USA
Mei You, College of Nursing, Michigan State University, East Lansing, MI, USA
Sangchoon Jeon, School of Nursing, Yale University, New Haven, CT, USA
Victoria Champion, School of Nursing, Indiana University, Indianapolis, IN, USA
Ruth McCorkle, School of Nursing, Yale University, New Haven, CT, USA
References
- 1.Clark NM. Management of chronic disease by patients. Annu Rev Public Health. 2003;24:289–313. doi: 10.1146/annurev.publhealth.24.100901.141021. [DOI] [PubMed] [Google Scholar]
- 2.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: A review. Patient Educ Couns. 2002;48:177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 3.Corbin JM, Strauss A. A nursing model for chronic illness management based upon the Trajectory Framework. Sch Inq Nurs Pract. 1991;5:155–174. [PubMed] [Google Scholar]
- 4.Lorig K, Holman HR, Sobel D, Laurent D, Gonzalez V, Minor M. Living a Healthy Life with Chronic Conditions. 2. Boulder: Bull Publishing; 2000. [Google Scholar]
- 5.Glasgow RE, Osteen VL. Evaluating diabetes education. Are we measuring the most important outcomes? Diabetes Care. 1992;15:1423–1432. doi: 10.2337/diacare.15.10.1423. [DOI] [PubMed] [Google Scholar]
- 6.Orem DE. The utility of self-care theory as a theoretical basis for self-neglect. J Adv Nurs. 2001;34:552–553. doi: 10.1046/j.1365-2648.2001.01831.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark NM. Asthma self-management education. Research and implications for clinical practice. Chest. 1989;95:1110–1113. doi: 10.1378/chest.95.5.1110. [DOI] [PubMed] [Google Scholar]
- 8.Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: Chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427–438. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 9.Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet. 2004;364:1523–1537. doi: 10.1016/S0140-6736(04)17277-2. [DOI] [PubMed] [Google Scholar]
- 10.Adler NE, Page AE, editors. Cancer Care for the Whole Patient. Washington: National Academic Press; 2008. [PubMed] [Google Scholar]
- 11.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive behavioral therapy: A review of meta-analysis. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Conn VS, Hafdahl AR, LeMaster JW, Ruppar TM, Cochran JE, Nielsen PJ. Meta-analysis of health behavior change interventions in type 1 diabetes. Am J Health Behav. 2008;32:315–329. doi: 10.5555/ajhb.2008.32.3.315. [DOI] [PubMed] [Google Scholar]
- 13.Bayliss EA, Ellis JL, Steiner JF. Barriers to self-management and quality-of-life outcomes in seniors with multimorbidities. Ann Fam Med. 2007;5:395–402. doi: 10.1370/afm.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sevick MA, Trauth JM, Ling BS, et al. Patients with complex chronic diseases: Perspectives on supporting self-management. J Gen Intern Med. 2007;22:438–444. doi: 10.1007/s11606-007-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barsevick AM, Sweeney C, Haney E, Chung E. A systematic qualitative analysis of psychoeducational interventions for depression in patients with cancer. Oncol Nurs Forum. 2002;29:73–84. doi: 10.1188/02.ONF.73-87. [DOI] [PubMed] [Google Scholar]
- 16.Crepez N, Passin WF, Herbst JH, et al. Meta-analysis of cognitive behavioral interventions on HIV-positive persons’ mental health and immune functioning. Health Psychol. 2008;27:4–14. doi: 10.1037/0278-6133.27.1.4. [DOI] [PubMed] [Google Scholar]
- 17.Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J Natl Cancer Inst. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen PB, Meade CD, Stein KD, Chirikos TN, Small BJ, Ruckdeschel JC. Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. J Clin Oncol. 2002;20:2851–2862. doi: 10.1200/JCO.2002.08.301. [DOI] [PubMed] [Google Scholar]
- 19.Yates P, Aranda S, Hargraves M, et al. Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2005;23:6027–6036. doi: 10.1200/JCO.2005.01.271. [DOI] [PubMed] [Google Scholar]
- 20.Sikorskii A, Given CW, Given B, et al. Symptom management for cancer patients: A trial comparing two multimodal interventions. J Pain Symptom Manage. 2007;34:253–264. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck AT. Cognitive therapy and emotional disorders. Arch Gen Psychiatry. 1976;41:113–114. [Google Scholar]
- 22.Dobson KS, Dozois DJA. Historical and philosophical bases of the cognitive-behavioral therapies. In: Dobson KS, editor. Handbook of Cognitive Behavioral Therapies. New York: The Guilford Press; 2001. pp. 3–39. [Google Scholar]
- 23.Persons JB, Davidson J. Cognitive-behavioral case formulation. In: Dobson KS, editor. Hand-book of Cognitive Behavioral Therapies. New York: The Guilford Press; 2001. pp. 86–110. [Google Scholar]
- 24.McGinn LK, Sanderson WC. What allows cognitive behavioral therapy to be brief: Overview, efficacy, and crucial factors facilitating brief treatment. Clin Psychol Sci Pract. 2001;8:23–37. [Google Scholar]
- 25.Hryniuk W, Ragaz J, Peters W. Dose density by any other name. J Clin Oncol. 2004;22:750–751. doi: 10.1200/JCO.2004.99.130. [DOI] [PubMed] [Google Scholar]
- 26.Czaja SJ, Schulz R, Lee CC, Belle SH. A methodology for describing and decomposing complex psychosocial and behavioral interventions. Psychol Aging. 2003;18:385–395. doi: 10.1037/0882-7974.18.3.385. [DOI] [PubMed] [Google Scholar]
- 27.Leventhal H, Friedman MA. Does establishing fidelity of treatment help in understanding treatment efficacy? Comment on Bellg et al. Health Psychol. 2004;23:452–456. doi: 10.1037/0278-6133.23.5.452. [DOI] [PubMed] [Google Scholar]
- 28.Given B, Given CW, McCorkle R, et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncol Nurs Forum. 2002;29:949–956. doi: 10.1188/02.ONF.949-956. [DOI] [PubMed] [Google Scholar]
- 29.Given CW, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22:507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 30.Given CW, Given B, Rahbar M, et al. Does a symptom management intervention affect depression among cancer patients: results from a clinical trial. Psychooncology. 2004;13:818–830. doi: 10.1002/pon.807. [DOI] [PubMed] [Google Scholar]
- 31.Sikorskii A, Given C, Given B, Jeon S, McCorkle R. Testing the effects of treatment complications on a cognitive behavioral intervention for reducing symptom severity. J Pain Symptom Manage. 2006;32(2):129–39. doi: 10.1016/j.jpainsymman.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Given B, Given CW, Sikorskii A, You M, McCorkle R, Champion V. Analyzing a symptom management trials: The value of both intention-to-treat and per protocol approaches. Oncol Nurs Forum. 2009;36:E293–302. doi: 10.1188/09.ONF.E293-E302. [DOI] [PubMed] [Google Scholar]
- 33.Sikorskii A, Given C, You M, Jeon S, Given B. Response analysis for multiple symptoms revealed differences between arms of a symptom management trial. J Clin Epidemiol. 2009;62:716–724. doi: 10.1016/j.jclinepi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Given CW, Sikorskii A, Tamkus D, et al. Managing symptoms among patients with breast cancer during chemotherapy: Results of a two-arm behavioral trial. J Clin Oncol. 2008;26:5855–5862. doi: 10.1200/JCO.2008.16.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate, and severe scores for cancer-related symptoms: How consistent and clinically meaningful are interference-based severity cut-points? J Pain and Symptom Manage. 2008;35:126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon S, Given CW, Sikorskii A, Given B. Do interference-based cut-points differentiate mild, moderate, and severe levels of 16 cancer-related symptoms over time? J Pain Symptom Manage. 2009;37:220–232. doi: 10.1016/j.jpainsymman.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cancer related fatigue version 1. [Access verified September 16, 2009]; doi: 10.6004/jnccn.2003.0029. Available at http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf. [DOI] [PubMed]
- 38.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 40.Bandura A. Self-Efficacy in Changing Societies. New York: Cambridge University Press; 1995. [Google Scholar]
- 41.Bandura A. Self-Efficacy. The Exercise of Control. New York: Freeman; 1997. [Google Scholar]
- 42.D’Zurilla T, Nezu A. Problem-solving strategies. In: Dobson K, editor. Handbook of Cognitive-Behavioral Therapies. New York: Guilford Press; 2001. pp. 211–245. [Google Scholar]
- 43.Nezu A, Nezu C, Houts P, et al. Relevance and problem solving therapy to psychosocial oncology. J Psychosoc Oncol. 1999;16:5–26. [Google Scholar]
- 44.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 45.Wang XS, Fairclough DL, Liao Z, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol. 2006;24:4485–4491. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]
- 46.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]