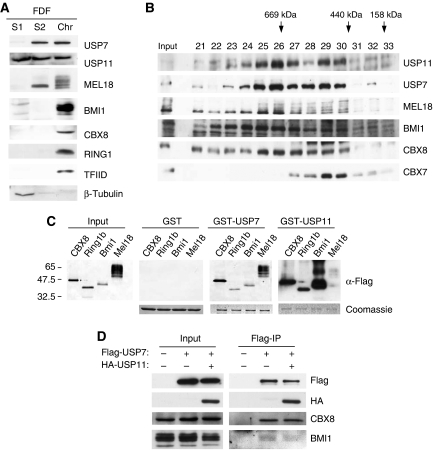
Figure 3.
Association of USP7 and USP11 with chromatin and high-molecular weight PRC1-like complexes. (A) Primary human fibroblasts (FDF) were used to prepare cytosolic (S1), nucleoplasmic (S2) and chromatin fractions (Chr) after standard procedures (Wysocka et al, 2001). Equivalent amounts of each fraction were separated by SDS–PAGE and immunoblotted for the indicated proteins. TFIID was used as a control for chromatin and β-tubulin as a non-chromatin protein. (B) Nuclear extracts (∼2 mg) from FDF cells were subjected to gel filtration on a Superose 6 column. Fractions (0.5 ml) were TCA precipitated, separated by SDS–PAGE and immunoblotted for the indicated proteins. Input refers to a sample of the cell extract before gel filtration and numbers above the gel specify relevant fraction numbers. Molecular weight standards (as indicated) were analysed separately on the same column and under the same conditions. (C) GST-pull-down assays using GST alone, GST-USP7 or GST-USP11 as indicated incubated with FlagHis12-tagged mouse Ring1b, Bmi1 and Mel18 produced from baculovirus vectors or human CBX8 expressed in E. coli. The left-hand panel shows the relative input levels of the different target proteins. The Coomassie-stained images below the panels confirm that equal amounts of recombinant protein were present in each pull-down assay. The PRC1 proteins were detected using a rabbit anti-Flag antibody and either alkaline phosphatase-conjugated anti-rabbit IgG (Input, GST- and GST-USP7-bound proteins) or horse radish peroxidase-conjugated anti-rabbit IgG (GST-USP11-bound proteins) as described in Materials and methods. (D) Co-precipitation of ectopically expressed Flag-USP7 and HA-USP11 from nuclear extracts of 293T cells. Endogenous CBX8 and BMI1 served as positive controls.