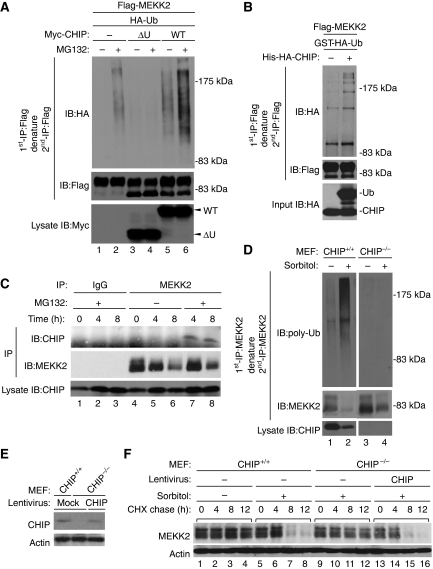
Figure 3.
CHIP is required for the proteasomal degradation of MEKK2 in response to sorbitol. (A) CHIP-dependent ubiquitination of MEKK2. HEK293 cells were transfected with the indicated combination of Flag-MEKK2WT, Myc-CHIPWT, Myc-CHIPΔU and HA-Ubiquitin (HA-Ub). After 24 h, cells were incubated with or without 100 nM MG132 for 18 h. Flag-MEKK2 was immunoprecipitated with anti-Flag antibody and re-immunoprecipitated with anti-Flag antibody after denaturation with 1% SDS followed by immunoblotting with indicated antibodies. The expression levels of Myc-CHIPWT and Myc-CHIPΔU were confirmed by immunoblotting with anti-Myc antibody. (B) In vitro ubiquitination of MEKK2 by CHIP. Flag-MEKK2WT transfected into HEK293 cells was purified with anti-Flag antibody followed by in vitro ubiquitination assay using ATP and recombinant proteins of E1, E2, GST-HA-Ub, His-HA-CHIP and Hsp70. Samples were analysed as described in (A). The protein amount of GST-HA-Ub and His-HA-CHIP was confirmed by immunoblotting with anti-HA antibody (Input). (C) Sorbitol-induced endogenous interaction of MEKK2 with CHIP. Cell lysates from MEFs treated with 300 mM sorbitol with or without 100 nM MG132 were immunoprecipitated with anti-MEKK2 antibody or control rabbit IgG followed by immunoblotting with indicated antibodies. (D) Sorbitol-induced endogenous ubiquitination of MEKK2 by CHIP. CHIP+/+ or CHIP−/− MEFs were pre-cultured in medium containing 0.1% FBS for 24 h and treated with or without 300 mM sorbitol in the presence of 100 nM MG132 for 12 h. Endogenous MEKK2 was immunoprecipitated with anti-MEKK2 antibody and re-immunoprecipitated with anti-MEKK2 antibody after denaturation with 1% SDS. Ubiquitinated MEKK2 was detected by anti-polyubiquitin antibody. The protein amount of MEKK2 and CHIP was confirmed by immunoblotting with anti-MEKK2 and CHIP antibodies. (E, F) Requirement of CHIP for sorbitol-induced MEKK2 degradation. CHIP+/+ or CHIP−/− MEFs were infected with lentivirus encoding CHIP or mock virus. After 48 h, MEFs pre-cultured in DMEM containing 0.1% FBS for 24 h were treated with or without 300 mM sorbitol in the presence of 100 μg/ml cycloheximide (CHX). The protein amount of MEKK2 and actin was confirmed by immunoblotting with anti-MEKK2 and actin antibodies. Endogenous and lentivirus-mediated expression of CHIP was detected by immunoblotting with anti-CHIP antibody (E).