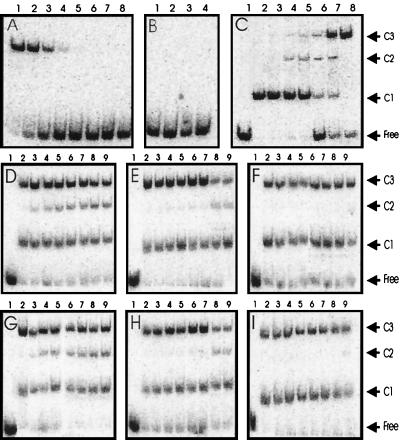
Figure 5.
Gel mobility shift assays with MBP-TraR. (A) MBP-TraR–AAI complexes in the following concentrations: 100, 30, 10, 3, 1, 0.3, 0.1, and 0.03 nM. (B) Lane 1, no protein added; lane 2, 1 μM apo-MBP–TraR without AAI; lane 3, 1 μM apo-TraR after incubation with 10 μM AAI for 4 h; lane 4, 1 μM apo-TraR after dialysis in the presence of 3% Tween and 10 μM AAI, followed by dialysis against 0.01% Tween and 10 μM AAI. (C) Gel mobility shifts by mixtures of TraR-AAI and MBP-TraR–AAI. TraR was incubated with MBP-TraR (both complexed with AAI) overnight, combined with DNA fragments, and size-fractionated. Lane 1, no protein; lanes 2–8, TraR and MBP-TraR combined at ratios of 1:0, 1:0.1, 1:0.33, 1:1, 1:3, 1:10, and 0:1, respectively. The total protein concentration was 20 nM. (D) TraR and MBP-TraR (500 nM each) were combined in binding buffer at 28°C for 0, 2, 4, 6, 8, 10, 12, and 14 h (lanes 2–9, respectively) and then stored at −20°C. Samples were thawed and combined with DNA at 0°C for 20 min and size-fractionated. (E and F) Similar to D, except that proteins were combined at 16°C and 4°C, respectively. (G–I) Similar to D–F, respectively, except that proteins were combined at concentrations of 100 nM. Free, unbound DNA; C1, DNA complexed with TraR homodimers; C2, DNA complexed with TraR–MBP-TraR heterodimers; C3, DNA complexed with MBP-TraR homodimers.