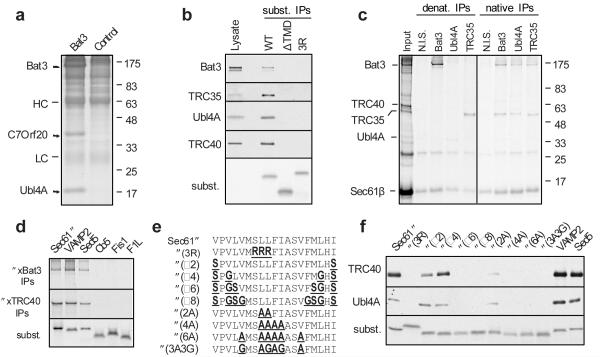
Fig. 1. Identification of a TMD-interacting protein complex.
a, Cytosolic proteins bound and eluted from anti-Bat3 or anti-GFP (control) affinity columns are shown. HC and LC are IgG heavy and light chain. b, Sec61β (WT), a deletion construct lacking its TMD (ΔTMD), or the insertion-deficient 3R mutant were translated in reticulocyte lysate, affinity purified on an anti-Sec61β column, and immunoblotted for the indicated products. Total lysate was included for comparison. An autoradiograph of the blot revealed equal recovery of the three translated substrates. c, Crosslinking products of Sec61β (from pooled sucrose gradient fractions 6-9 in Sup. Fig. S4) were immunoprecipitated under denaturing or native conditions. Non-immune serum (N.I.S) was included as a control. d, Versions of Sec61β containing the TMD from the indicated proteins were analyzed for interaction with Bat3 and TRC40 by in vitro translation, crosslinking, and immunoprecipitation. An aliquot of the total translation reaction is shown for each substrate (‘subst.’), as well as the immunoprecipitation products of the crosslinking reactions. e, The TMD of Sec61β was mutated to change its hydrophobicity as indicated. f, Each construct was analyzed for its interactions with Bat3 complex and TRC40 as in panel b. An aliquot of the total translation product was analyzed by autoradiography to visualize the substrates.