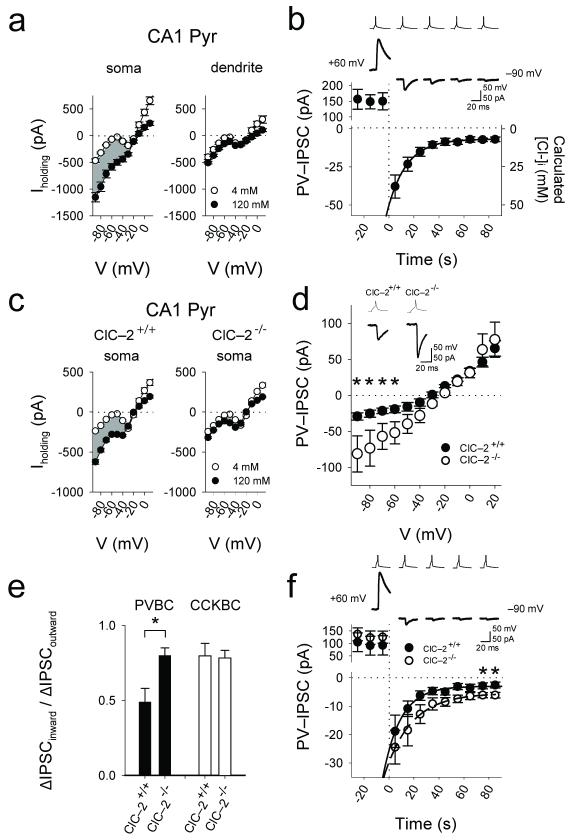
Figure 2. Somatic hyperpolarization-gated, sustained Cl−-conductance mediated by ClC–2.
(a) Whole-cell recordings from the somata and proximal apical dendrites of pyramidal cells with different [Cl−]pip (4mM: nsoma=14, ndendrite=4; 120mM: nsoma=11, ndendrite=4; shading indicates the difference current reflecting whole-cell Cl− current). (b) Time-dependent decrease of PV-IPSCs after stepping the membrane voltage of the postsynaptic cell from +60 mV to −90 mV in rat. (c) Large sustained somatic Cl−-conductance in somata of CA1 pyramidal cells in the wild-type (ClC–2+/+) but not the ClC–2−/− mice (4mM: nsoma,+/+ =13, nsoma,−/− =20; 120mM: nsoma,+/+ =24, nsoma,−/− =24). (d) Current-voltage plots of PV-IPSCs from ClC–2+/+ (n=6 pairs) and ClC–2−/− (n=3 pairs). Inset: PV-IPSCs from ClC–2−/− mice compared to ClC–2+/+ (example traces at −70 mV; [Cl−]pip=40 mM). (e) Significantly decreased outward rectification of the PV-IPSCs in the ClC–2−/− mice, and lack of change in rectification in the case of CCK-IPSCs. (f) Slower time-dependent decrease of PV-IPSCs after stepping the membrane voltage of the postsynaptic cell from +60 mV to −90 mV in the ClC–2−/− mice compared to ClC–2+/+. Asterisks indicate significant difference (note that the larger IPSCs indicated by asterisks in these Cl− extrusion experiments are in general agreement with the presence of larger IPSCs at hyperpolarized holding potentials in the ClC–2−/− animals in Fig. 2d).