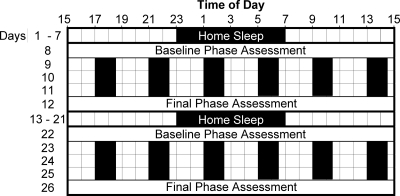
Figure 1.
Diagram of the protocol. Subjects slept on a fixed sleep schedule (8 to 9 h per night) tailored to their habitual sleep times for 7 d (example of 2300 to 0700 h shown in figure), and then participated in a laboratory session for 5 d. Subjects returned to sleeping on the fixed sleep schedule for 9 d before returning for a second laboratory session. During the phase assessments, on d 8, 12, 22, and 26, saliva was sampled every 30 min in dim light (<5 lux) to measure the entire melatonin profile and calculate the DLMOs. There were 3 d of an ultradian light/dark cycle during each laboratory session in which subjects lay on cots in the dark (0 lux) for 1.5 h, were permitted to sleep (represented by black bars), and were kept awake for 2.5 h in room light [<150 lux, 37.3 ± 13.8 lux (mean ± sd)] and were permitted to eat and drink ad libitum. For each of the 3 ultradian days, subjects were given a pill at the start of one of the wake episodes, thus at the same clock time each day. During one laboratory session, the pill was melatonin (0.5 mg immediate release), and during the other session it was placebo, counterbalanced, double-blind. Different groups of three to four subjects received the pills at the start of different awake episodes to cover the entire 24-h day. The phase shift during each laboratory session was the baseline DLMO minus the final DLMO. The phase shift (free-run) during the placebo session was subtracted from the phase shift during the melatonin session and plotted against the time of melatonin administration relative to baseline DLMO or relative to home sleep midpoint to generate the PRCs.