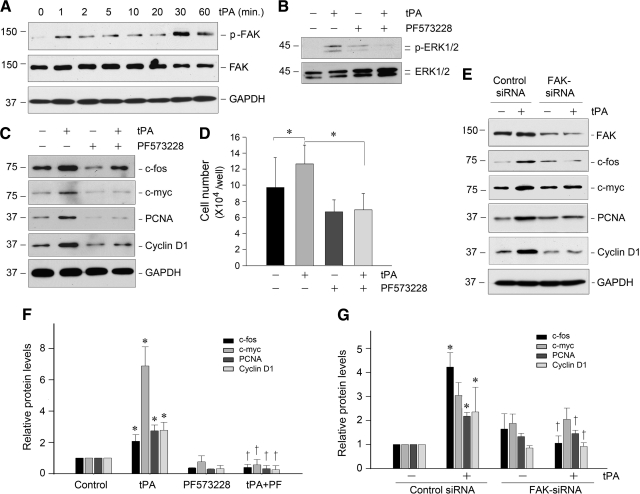
Figure 7.
tPA induces renal fibroblast proliferation by a FAK-dependent mechanism. A: tPA induced a rapid FAK tyrosine phosphorylation and activation in renal fibroblasts. NRK-49F cells were treated with tPA (20 nmol/L) for various periods of time as indicated. Cell lysates were immunoblotted with specific antibodies against phospho-FAK, total FAK, and GAPDH, respectively. B: tPA-mediated ERK1/2 activation was dependent on FAK signaling. NRK-49F cells were pretreated with specific FAK inhibitor PF573228 (10 μmol/L) for 30 minutes, followed by incubation with tPA. C: Blockade of FAK activation abolished the expression of proliferation-related genes induced by tPA. NRK-49F cells were treated as indicated, and cell lysates were subjected to Western blot analyses for c-fos, c-myc, PCNA, cyclin D1, and GAPDH, respectively. D: Blockade of FAK signaling abrogated tPA-mediated cell proliferation. *P < 0.05 (n = 3). E: Knockdown of FAK abolished tPA-mediated induction of various proliferation-related genes. NKR49F cells were transfected with either FAK-specific siRNA or control siRNA, followed by incubation with or without tPA (20 nmol/L) for 48 hours, respectively. Cell lysates were immunoblotted with specific antibodies as indicated. F and G: Quantitative data show the proliferation-related protein abundances after various treatments as shown in C and E, respectively. Relative protein levels (fold induction over controls) were determined by densitometric analyses and reported after normalizing with GAPDH. Data were means ± SEM of three experiments. *P < 0.05 versus controls; †P < 0.05 versus tPA alone (F), or versus control siRNA (G).