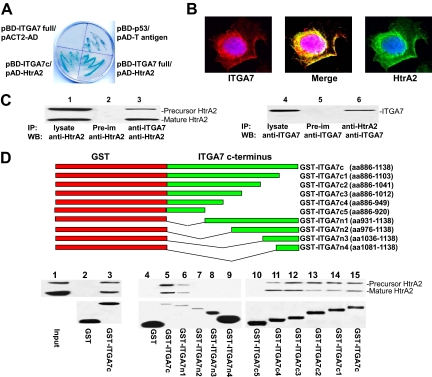
Figure 1.
The C-terminus of integrin α7 interacts with HtrA2. A: β-galactosidase activity of yeast harboring pBD-ITGA7 full and pACT2-AD (negative control), pBD-ITGA7c and pAD-HtrA2, pBD-p53 and pAD-T antigen (positive control), or pBD-ITGA7 full and pAD-HtrA2. B: ITGA7 and HtrA2 co-localized in PITT1 cells. PITT1 cells were induced to express ITGA7 with 5 μg/ml tetracycline for 24 hours. Immunostaining was performed by using antibodies specific for ITGA7 or HtrA2 as described in Materials and Methods. C: Co-immunoprecipitation of ITGA7 and HtrA2. PITT1 cells were induced with 5 μg/ml tetracycline for 24 hours. The lysates were immunoprecipitated with the indicated antibodies and detected by Western blotting with either ITGA7 or HtrA2 antibodies. D: ITGA7 binds with HtrA2 in vitro. Upper panel: Diagrams of GST-ITGA7c mutants. Middle panel: Binding of GST-ITGA7c fusion proteins and its mutants with HtrA2 from PITT1 cells. After extensive washes, the bound proteins were eluted and immunoblotted with anti-HtrA2 antibodies. Lower panel: Coomassie staining of fusion proteins from the middle panel.