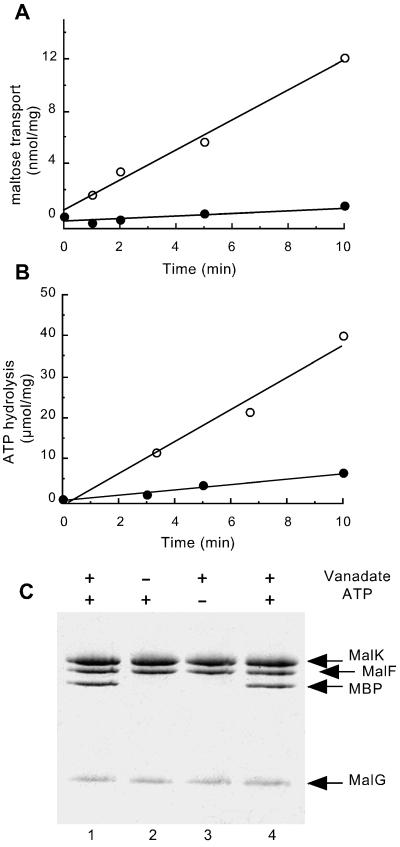
Figure 1.
Analysis of vanadate-treated proteoliposomes. Purified MalFGK2 (2.5 μM) (18), reconstituted in proteoliposomes, was incubated with 5 μM purified MBP (21), 14 mM MgCl2, 4 mM ATP, and 0.1 mM maltose for 20 min at 37°C in the presence (●) or absence (○) of 0.5 mM vanadate. Proteoliposomes were then diluted 20-fold in 20 mM Hepes (pH 8)/0.1 mM EDTA, collected by centrifugation, and suspended in Hepes/EDTA buffer. (A) [14C]Maltose uptake into proteoliposomes (see Materials and Methods). (B) ATPase activity (see Materials and Methods) is measured on the same freeze/thawed proteoliposome preparation that was used for the transport measurements. (Proteoliposomes were washed by centrifugation to remove excess unlabeled ATP before assay.) (C) The protein content of proteoliposome pellets is visualized by Coomassie staining after SDS/PAGE. Lanes 1–3: Proteoliposomes incubated in the presence or absence of vanadate and ATP as indicated. Lane 4: Sample treated as in lane 1, but with 20 μM MBP.