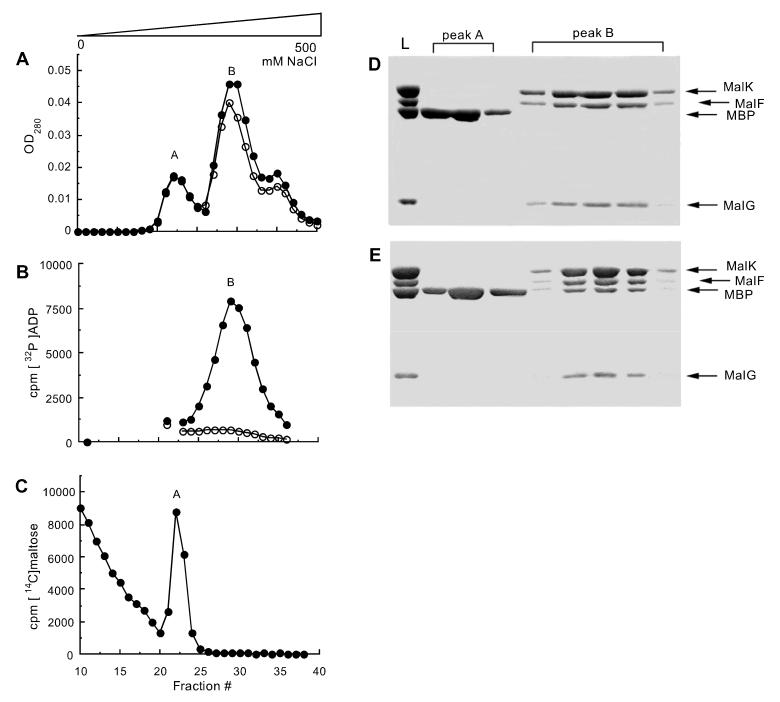
Figure 2.
Formation of a stable complex between MBP and MalFGK2 in detergent solution. MalFGK2 (2.5 μM) in 20 mM Hepes (pH 8), 10% glycerol, and 0.01% n-dodecyl-β-d-maltoside (exhibiting 0.2 μmol/min/mg of ATPase activity) was incubated with 5 μM MBP, 4 mM MgCl2, and 4 mM ATP for 20 min at 37°C in the presence (●) or absence (○) of 0.5 mM vanadate, then loaded onto a HiTrap Q Sepharose column (Amersham Pharmacia) and eluted with a gradient of NaCl. (A) OD280 of fractions eluting during the gradient. (B) Radioactive elution profile of a sample incubated in the presence of 1 mM MgCl2 and 0.8 mM [α-32P]ATP in place of unlabeled ATP. The sample is desalted before ion exchange. (C) Radioactive elution profile of a sample incubated in the presence of 0.5 mM vanadate and 10 μM [14C]maltose in place of unlabeled maltose. The sample is desalted before ion exchange. (D) Protein composition of fractions from a sample incubated in the absence of vanadate, visualized by Coomassie staining after SDS/PAGE. L, loaded sample. (E) Protein composition of fractions from a sample incubated in the presence of vanadate.