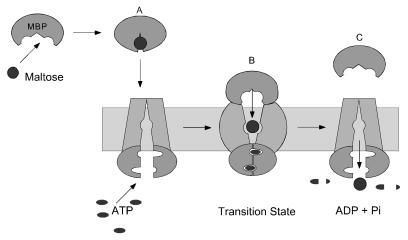
Figure 4.
A model for maltose transport. (A) MBP binds maltose and undergoes a conformational change from an open to a closed conformation, generating a high-affinity sugar-binding site. In the closed conformation, MBP binds MalFGK2 to initiate transport and hydrolysis. (B) In the transition state, MBP and MalFGK2 become tightly bound to each other, and both proteins have opened, exposing internal binding sites to each other. Opening of MBP in the transition state weakens the interaction between MBP and maltose, facilitating the transfer of sugar to the low-affinity binding site in MalFGK2. (C) Maltose is transported and MBP is released after the re-exposure of the internal binding site to the cytoplasm. The MalK subunits are modeled after the Rad50 catalytic domain structure (33). This ABC protein undergoes an ATP-induced dimerization and activation step that completes both nucleotide-binding sites with residues donated from the opposing subunit. By analogy, MBP may stimulate the ATPase activity of MalK by bringing the two subunits into close proximity in the transition state.