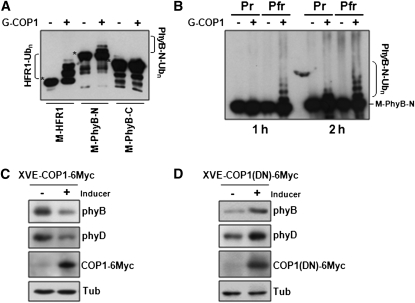
Figure 2.
In Vitro Ubiquitination of PhyB by COP1 E3 Ligase.
(A) COP1-mediated ubiquitination of PhyB N-terminal domain. MBP fusions with HFR1 or the N- or C- terminal domains of PhyB were incubated with E1 ubiquitin activating enzyme and E2 ubiquitin conjugating enzyme in the presence or absence of GST-COP1 (G-COP1). Immunoblots of the reactions were probed with anti-MBP. Asterisks indicate the original size of M-HFR1, M-PhyB-N, and M-PhyB-C.
(B) COP1 preferentially ubiquitinates Pfr of phyB-N. MBP-phyB-N reconstituted with the chromophore PCB was used as substrate in in vitro ubiquitination assays. In vitro ubiquitination assay was performed at 30°C for 1 h or 2 h with Pfr and Pr forms of MBP-fused phyB-N (M-phyB-N).
(C) Immunoblots showing decreased phyB and phyD levels in transgenic plants after induction of COP1 expression under R light.
(D) Immunoblots showing increased phyB and phyD levels after inducing the expression of a dominant-negative mutant COP1(DN) under R light.
In (C) and (D), phyB, phyD, and COP1 levels were detected by anti-phyB (BA2), anti-phyD, and anti-Myc antibodies, respectively. Tubulin (Tub) was detected as a loading control.