Figure 8.
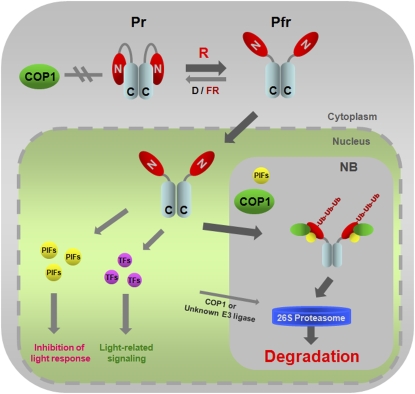
A Model for Light-Dependent Proteasomal Degradation of phyB.
In D or FR light, phyB remains in the cytosol as the inactive Pr form in which both the N-terminal (GAF-PHY domains) and C-terminal regions (PRD) are able to interact (Kircher et al., 1999; Yamaguchi et al., 1999; Chen et al., 2003, 2005). This particular conformation might prevent the association between COP1 and phyB, resulting in a stable phyB that accumulates in the cytosol. However, upon R light exposure, the NLS, located at the C-terminal region of phyB, is exposed due to a light-dependent conformational change (Chen et al., 2005). Therefore, the active Pfr form translocates into nuclei where it triggers signaling by binding to PIFs or other transcription factors (TFs). PIFs may promote phyB polyubiquitination by increasing the binding affinity between phyB and COP1 and thus facilitating phyB degradation by 26S proteasomes. Most probably the phyB-PIFs-COP1 interaction takes place in nuclear bodies (NB), subnuclear structures where COP1 colocalizes with several of its targets (LAF1, HY5, HFR1, and phyA) (Saijo et al., 2003; Seo et al., 2003, 2004; Jang et al., 2005; Yang et al., 2005), since phyB also colocalizes to NB in a PIF3-dependent manner (Bauer et al., 2004). It has been proposed that phyB and PIFs negatively regulate each other (Monte et al., 2007; Al-Sady et al., 2008; Henriques et al., 2009). Although we provide evidence for the PIF-mediated downregulation of phyB levels, the exact mechanism for light-induced and phyB-dependent PIF degradation remains to be characterized.
[See online article for color version of this figure.]