Table 1.
Amination of allyl alcohol (2 equiv) as a function of nucleophile catalyzed by a mixture of (2)AuCl (5 mol %) and AgSbF6 (5 mol %) in dioxane.
| entry | nucleophile | product | temp (°C) | time (h) | yield (%)a |
|---|---|---|---|---|---|
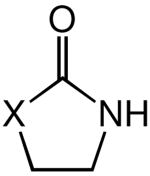
|
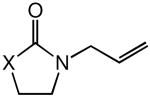
|
||||
| 1 | X = NMe (1) | 60 | 2 | 99b | |
| 2 | X = O | 100 | 48 | 98 | |
| 3 | X = CH2 | 80 | 48 | 42 | |
| 4 |
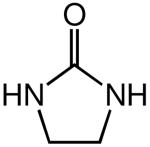
|
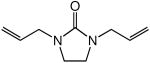
|
60 | 24 | 100 |
| 5 | TsNMeH |
|
100 | 24 | 99 |
| RNH2 |
|
||||
| 6 | R = Ts | 100 | 48 | 78 | |
| 7 | R = 4-MeOC6H4SO2 | 100 | 24 | 80c | |
| 8 | R = Cbz | 100 | 72 | 23 |
Isolated material of >95% purity.
One equivalent of allyl alcohol employed.
N,N-Diallyl-4-methoxybenzenesulfonamide was also isolated in 20% yield.
