“It is only through labor and painful effort, by grim energy and resolute courage, that we move on to better things.”
—Theodore Roosevelt
Neonatal drug therapy: first things first
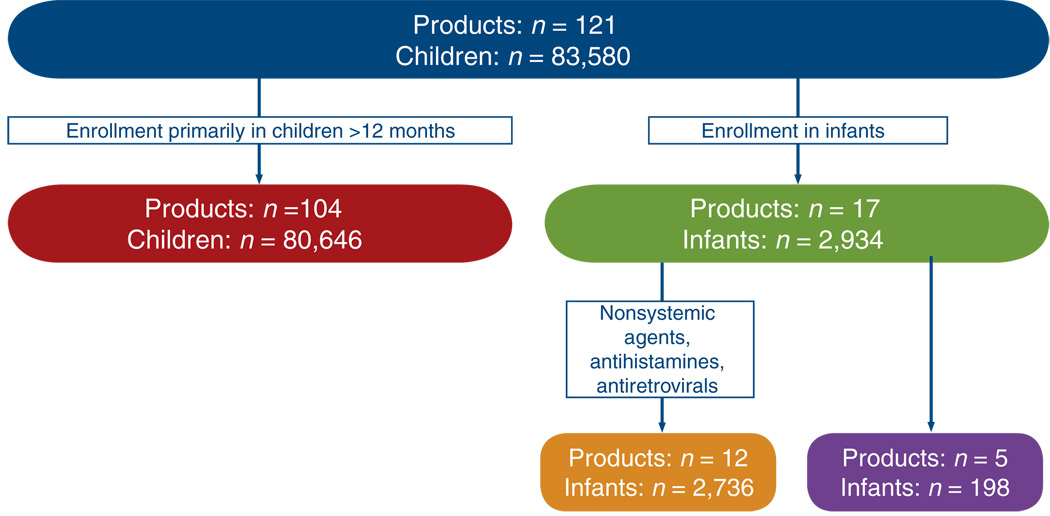
Legislation enacted in the United States over the past 12 years has had a critical role in providing dose and safety data for a large number of drugs used in children. The success of these efforts cannot be undervalued; more than 300 labeling changes have occurred during this time period. However, significant gaps remain in our knowledge of drug disposition in the infant and neonate, and the existing legislation guiding pediatric drug development in the United States has not addressed the need for developing sound therapeutic strategies for the most vulnerable members of society. The majority of children enrolled in trials submitted to the US Food and Drug Administration (FDA) under the Best Pharmaceuticals Act have been older than 12 months of age (Figure 1). Excluding antiretrovirals, antihistamines, and topical agents, only five agents were the focus of trials designed primarily to obtain pharmacokinetic data in children in this age group. Thus, as further addressed by Ward and Kern in this issue,1 neonates continue to represent the “therapeutic orphan”2 in clinical pharmacology today, and neonatal pharmacology represents a discipline with numerous challenges before it. This issue of the Journal highlights those challenges, but also, more importantly, it illustrates evolving approaches that have been successful in meeting these challenges.
Figure 1.
Age of subjects enrolled in pediatric studies (1998–2005) contributing to market exclusivity. Of the five products for which a study was conducted entirely in infants, only ranitidine is commonly used in neonatal care.18 Courtesy of Brian Smith and Danny Benjamin.
Optimal treatment of serious fungal infections in preterm neonates is a recurring theme throughout the issue. This therapeutic example illustrates the existing limitations in our current knowledge base as well as recent efforts to fill knowledge gaps. Worldwide, fungal infections (Candida spp.) continue to be a major cause of morbidity and mortality for low-birth-weight neonates. There is a 16% incidence of candidemia in neonates with a birth weight under 750 g (ref. 3). Treatment of these infections varies among centers and around the world, but first-line therapy typically includes the use of one of the following: amphotericin B deoxycholate, lipid complex amphotericin, or fluconazole. None of these drugs has received FDA approval in the United States for use in this population, but some pharmacokinetic data (albeit incomplete) exist to guide therapy. Some speculate that lipid complex amphotericin products may be a better option than amphotericin B deoxycholate for ill premature neonates because of their more favorable adverse-event profiles. However, the maximum dose of these drugs (trade names: Abelcet, AmBisome) and the optimal duration of treatment have not been defined.
Fluconazole is increasingly used in the neonatal intensive care unit. Two articles in this issue cite recent data generated regarding the optimal dosing of fluconazole in this population and the use of sparse sampling strategies and population pharmacokinetic models to generate these data.4,5 Importantly, the data demonstrate that previously recommended doses for fluconazole were inadequate for the eradication of fungal infections in preterm neonates. The efficacy and need for antifungal prophylaxis remains an unresolved issue, and ongoing studies will address the efficacy of this approach. Thus, for premature neonates born around the world, the basic clinical pharmacology data needed to define the appropriate dose, safety, and duration of therapy for many anti-infectives (as well as other compounds) are incomplete.
Another emphasis of this issue of the Journal is the critical need for multicenter trials for the elucidation of pharmacokinetic, pharmacodynamic, safety, and efficacy data in neonatal pharmacology.1,6 The success of pediatric oncology clinical trial groups provides a model that could be followed to achieve safe and rational therapeutics for neonates. Moran and colleagues address issues related to the effect that inter-center differences can have on the selection of appropriate end points for future clinical trials in neonatal pharmacology.6
The “in the trenches” challenges associated with clinical pharmacology studies in ill neonates cannot be overlooked. As addressed by Ward and Kern,1 clinical pharmacology studies performed in children less than a year of age entail a unique set of obstacles. Difficulties related to parental consent, the ethics of performing research on vulnerable subjects, limitations in blood volume sampling, and the development of age-appropriate pharmacodynamic end points are some of these obstacles. In recent years, the development of microsampling techniques and sensitive analytical methods has facilitated clinical trials in neonates. In addition, studies using sparse sampling and population pharmacokinetic models are increasingly used in neonatal pharmacology.
Another approach is the use of in silico simulation to forecast experimental outcomes. Simulation based on pharmacokinetic models, if properly constructed, can be used to acquire an initial estimate of an appropriate drug dose and dosing interval to achieve a target serum concentration range in the patient population in question. Mouksassi et al. demonstrate this approach in pediatric trial design using pharmacokinetic data generated in adults for the drug teduglutide.7 This compound, a synthetic glucagon-like peptide-2 analog, is being studied for use in the treatment of short-bowel syndrome, which may occur in neonates as a consequence of necrotizing enterocolitis, malrotation, or intestinal atresia.
Intrauterine drug exposure: pharmacogenetic approaches to assess fetal risk
A previous issue of the Journal8 focused on women’s health issues and highlighted the need for better safety information for drugs being utilized in pregnant women, particularly concerning safety aspects of drug exposure for the developing fetus. Data suggest that more than 60% of pregnant women receive drug prescriptions during their pregnancies.9 Study of the interplay between drug exposure in utero and possible genetic predisposition for the development of drug-induced birth defects may elucidate new mechanistic paradigms for the development of birth defects. In this issue, Leeder10 describes a systematic framework for identifying candidate genes involved in drug-induced birth defects. A critical component of this approach is consideration of the role of development in the expression of drug-metabolizing enzymes and methodically profiling the switch from fetal to adult forms of mechanistically relevant drug metabolism pathways. Although acetaminophen exposure in utero conveys only a modest risk for the development of gastroschisis, a rare congenital defect affecting the abdominal wall, the co-exposure of acetaminophen and pseudoephedrine amplifies this risk by more than twofold. Understanding the metabolism of acetaminophen in the fetal liver may shed light on this observation. The metabolism of acetaminophen in adults is mediated primarily by SULT1A1, UGT1A6, and UGT1A9; however, UGT1A6 is absent in fetal liver, and the metabolism of acetaminophen by sulfation therefore plays a predominant role in the fetus.11 Studies in fetal liver reveal unique sulfation enzyme expression patterns as well as transition periods in the expression of fetal and adult forms of sulfation isoforms. In addition to drug metabolism, sulfation in the fetus may serve transporter functions and have a role in vasoactive catecholamine metabolism.10 Because of these other biological effects, genetic variability in candidate SULTs supports the further study of these candidate genes in association with the development of drug-induced birth defects.
Intrauterine drug exposure and neurobehavioral consequences
Maternal depression is a common clinical condition that affects approximately 1 in 10 women of childbearing age. Selective norepinephrine or serotonin reuptake inhibitors (SSRIs) are the primary drugs used for the treatment of depression in pregnant women, in large part because of their favorable safety profiles for both the mother and fetus. Early studies examining potential risks for the development of birth defects with exposure to these drugs have been reassuring,11 although some data suggest possible effects on the development of the fetal pulmonary vasculature.12,13 Initial reports of favorable safety profiles further propel use of these drugs during pregnancy. Oberlander et al.14 describe efforts to examine the effect of in utero exposure to SSRIs on neurobehavioral outcomes. The issue is complex because antenatal stress alone has been linked to adverse neurobehavioral outcomes in children. Some data suggest that neonates exposed to SSRIs in utero have blunted responses to pain stimuli.15 Oberlander and colleagues14 discuss data in support of the hypothesis that SSRI exposure in utero leads to elevated concentrations of fetal 5-hydroxytryptamine, which may affect serotonin regulation and feedback in the developing fetus and lead to adverse neurobehavioral effects in the child in later life.
Conclusion
“A little more persistence, a little more effort, and what seemed hopeless failure may turn to glorious success.”
—Elbert Hubbard
Lessons from history teach us that “trial and error” approaches to drug dosing in sick neonates and young infants can have devastating consequences. Technological advances achieved over the past 30 years have resulted in improvements in our ability to resuscitate and sustain premature neonates. Future efforts to achieve effective and safe drug dosing paradigms for these vulnerable members of society will require multifaceted approaches. Regulatory programs that provide incentives to the pharmaceutical industry to examine drug disposition and potential adverse effects in neonates constitute an obvious initial approach that must be embraced and that has proved successful in generating new labeling information pertaining to older children.16
Recent changes in the legislation guiding pediatric drug development in Europe (i.e., European Union legislation) represent a high standard that should be applauded and modeled.17 Through this legislation, incentives to pharmaceutical companies for patentprotected drugs, off-patent drugs, and orphan drugs are provided. Studies for pediatric drug development are outlined in Pediatric Investigation Plans that are approved by the Pediatric Committee of the European Medicines Agency. As an example of the breadth of this new legislation and its implications for children, infants, and neonates, pharmaceutical companies must generate new pediatric data with every new indication that is filed for a drug. In addition, up to 12 years of market exclusivity is available to pharmaceutical companies that have pediatrics-based orphan drug development programs. Results of all pediatric studies will be publicly available, thus assuring the dissemination of pharmacokinetic data for drugs used in children.17 In addition to enhanced regulatory mandates, federal research programs in the United States and around the world that make neonatal drug research a priority can also provide a needed stimulus to enhance the existing knowledge base. Ultimately, the creation of neonatal drug development programs and the future development of safe and effective drug therapy for neonates must be a partnership that is supported by federal mandates and achieved through the synergistic efforts of clinical pharmacologists and neonatologists in academia working in concert with industry.
ACKNOWLEDGMENT
The editorial comments of Brian Smith and Danny Benjamin are gratefully acknowledged.
Footnotes
CONFLICT OF INTEREST
L.J. has research support from the National Institutes of Health through the following grants: DK075936, DK079387, HD31324, and DK81406. S.I. has research support from the Canadian Institute for Health Research (MT13747 and MOP82907), AstraZeneca, and the Ministry of Health and Long-Term Care.
References
- 1.Ward RM, Kern SE. Clinical trials in neonates: a therapeutic imperative. Clin. Pharmacol. Ther. 2009;86:585–587. doi: 10.1038/clpt.2009.207. [DOI] [PubMed] [Google Scholar]
- 2.Shirkey HC, editor. Pediatric Therapy. St. Louis: C.V. Mosby; 1964. [Google Scholar]
- 3.Benjamin DK, Jr, Stoll BJ, Goldberg R, Walsh TJ for the NICHD Neonatal Research Network. Neonatal candidiasis: epidemiology, clinical judgment, and outcomes (abstract). Presented at the annual meeting of the Society for Pediatric Research; 2–6 May 2008; Honolulu, Hawaii. [Google Scholar]
- 4.Wade KC, et al. Population pharmacokinetics of fluconazole in young infants. Antimicrob. Agents Chemother. 2008;52:4043–4049. doi: 10.1128/AAC.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufman D, Boyle R, Hazen KD, Patric JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N. Engl. J. Med. 2001;345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 6.Moran C, Smith PB, Cohen-Wolkowiez MC, Benjamin DK., Jr Clinical trial design in neonatal pharmacology: effect of center differences with lessons from the pediatric oncology cooperative research experience. Clin. Pharm. Ther. 2009;86:589–591. doi: 10.1038/clpt.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouksassi MS, Marier JF, Cyran J, Vinks AA. Clinical trial simulation in pediatric patients using realistic covariates: application to teduglutide, a glucagon-like peptide-2 analog in neonates and infants with shortbowel syndrome. Clin. Pharmacol. Ther. 2009;86:667–671. doi: 10.1038/clpt.2009.199. [DOI] [PubMed] [Google Scholar]
- 8.Uhl K. Advancing women’s health in the 21st century: applying the tools of clinical pharmacology. Clin. Pharmacol. Ther. 2008;83:3–7. doi: 10.1038/sj.clpt.6100463. [DOI] [PubMed] [Google Scholar]
- 9.Andrade SE, et al. Prescription drug use in pregnancy. Am. J. Obstet. Gynecol. 2004;191:298–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Leeder JS. Developmental pharmacogenetics: a general paradigm for application to neonatal pharmacology and toxicology. Clin. Pharmacol. Ther. 2009;86:678–682. doi: 10.1038/clpt.2009.195. [DOI] [PubMed] [Google Scholar]
- 11.Einarson A, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am. J. Psychiatry. 2008;165:749–752. doi: 10.1176/appi.ajp.2007.07060879. [DOI] [PubMed] [Google Scholar]
- 12.Chambers CD, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N. Engl. J. Med. 2006;354:579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 13.Fornaro E, Li D, Pan J, Belik J. Prenatal exposure to fluoxetine induces fetal pulmonary hypertension in the rat. Am. J. Respir. Crit. Care Med. 2007;176:1035–1040. doi: 10.1164/rccm.200701-163OC. [DOI] [PubMed] [Google Scholar]
- 14.Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurodevelopmental effects of intrauterine exposure to SSRI antidepressants: molecular to clinical evidence. Clin. Pharmacol. Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- 16.Ward RM, Kauffman R. Future of pediatric therapeutics: reauthorization of BPCA and PREA. Clin. Pharmacol. Ther. 2007;81:477–479. doi: 10.1038/sj.clpt.6100109. [DOI] [PubMed] [Google Scholar]
- 17.Vanchiere C, Butler AS, Knutsen A. Addressing the Barriers to Pediatric Drug Development: Workshop Summary. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 18.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]