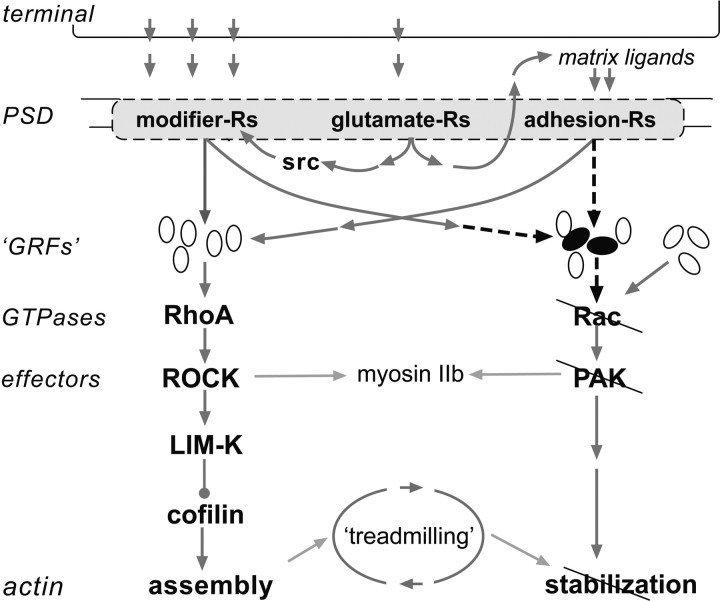
Figure 6.
Defects in physiologically driven actin signaling at hippocampal synapses in Fmr1-KO mice. Observed impairments and their hypothesized causes are represented in a summary diagram of events involved in the production of stable LTP. Three classes of postsynaptic receptors (-Rs) are engaged by theta bursts, two of which drive cytoskeletal modifications. Bound neurotransmitter receptors (glutamate-Rs) initiate events that promote the full activation of these two groups. The modifier-Rs stimulate RhoA, presumably via multiple GTPase-regulatory factors (GRFs), which then initiate a pathway that goes through multiple effectors to trigger actin filament assembly. The modifier-R for BDNF (i.e., TrkB) also facilitates Rac>PAK signaling, which drives unknown effectors to stabilize the newly formed filaments. Adhesion-Rs belonging to the β1 integrin family also drive the RhoA assembly cascade and are assumed from the literature to have potent effects on the Rac, stabilization pathway. Past studies showed that the RhoA-initiated sequence is intact in Fmr1-KO slices; the present findings indicate that physiological activation of Rac and PAK is impaired (slashed lines) in the mutants, and that this is accompanied by a loss of rapid stabilization of both newly formed actin filaments and LTP. Given the results for RhoA, cofilin, and actin polymerization, the defect is not likely to reside in the membrane receptors or their activation. The proposed alternatives are (1) a flaw in the steps leading from the receptors to Rac (dashed arrows) and/or (2) defects in Rac-specific GRFs engaged by the membrane Rs (black ovals). The schematic includes a group of intact Rac-specific GRFs that are not linked to the membrane Rs: these are suggested by the observation that baseline levels of activated Rac and PAK appear normal in Fmr1-KO slices. Finally, myosin IIb is included in the schematic because its regulatory kinase is a target of PAK; disruption of this linkage in the mutants could lead to impaired myosin motor responses to afferent activity, and thus to the abnormalities in PAK distribution described here.