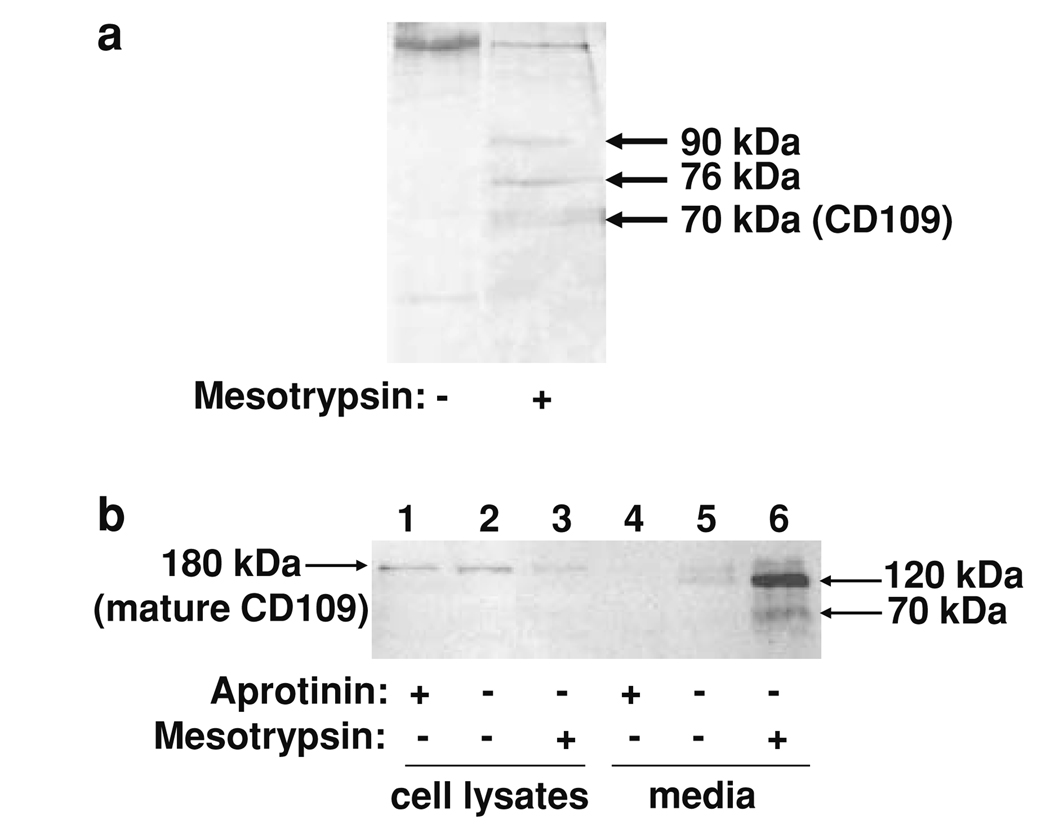
Fig. 5.
Identification of CD109 as a mesotrypsin proteolytic target. a Biotinylation and capture of cell surface proteins from conditioned medium of T4-2 cells yielded several silver-stained bands that were present after mesotrypsin treatment (right lane) but absent in an untreated control (left lane); the 70-kDa band was identified as CD109 by nanoLC-MS/MS. b T4-2 cells were grown in monolayer culture without treatment or in the presence of 100 µM aprotinin or 200 nM recombinant mesotrypsin, and then whole cell protein lysates and conditioned media were recovered and analyzed by western blotting using a sheep anti-human CD109 polyclonal primary antibody (R&D Systems, Minneapolis, MN, USA) at 0.15 µg/ml, and a rabbit anti-sheep HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:1,000 dilution. Immunostaining of cell lysates for CD109 was reduced when cells were treated with mesotrypsin (lane 3) relative to untreated cells (lane 2) or cells treated with aprotinin (lane 1). Conditioned medium from the mesotrypsin-treated cells contained 120 and 70 kDa CD109 fragments (lane 6). Conditioned medium from untreated cells showed evidence of limited CD109 shedding (lane 5), which was suppressed by aprotinin (lane 4)