Table 1.
Effect of Reaction Conditions on Carbonylative Conjugate Additions to Chalconea
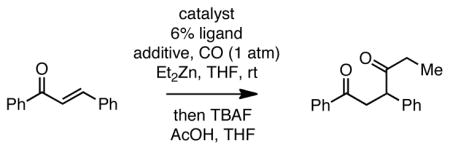 | ||||
|---|---|---|---|---|
| entry | catalyst | ligand | additive | % yield |
| 1 | 5% Pd(PPh3)4 | - | TMSCl+LiCl | 70 |
| 2 | 5% Pd(PPh3)4 | - | TMSCl | 82 |
| 3 | 5% Pd(PPh3)4 | - | - | N.R. |
| 4 | 2.5% Pd2(dba)3 | PPh3 | TMSCl | 85 |
| 5 | 2.5% Pd2(dba)3 | PCy3 | TMSCl | 82 |
| 6 | 0.25% Pd2(dba)3 | PPh3 | TMSCl | 86 |
| 7 | 0.25% Pd2(dba)3 | PCy3 | TMSCl | 86 |
| 8 | 2.5% Pd2(dba)3 | PCy3 | TMSCl | 83b |
| 9 | 5% Ni(cod)2 | PCy3 | TMSCl | N.R. |
| 10 | 5% Pt(dba)3 | PCy3 | TMSCl | 0 |
Reaction with 1.3 equiv Et2Zn, 2.3 equiv TMSCl, 1 atm CO, for 4 h. Subsequent to reaction, the mixture was quenched at 0 °C with acetic acid (1.5 equiv) and TBAF (1.5 equiv) for 10 minutes and then treated with saturated Na2CO3.
Reaction with 0.65 equivalents of Et2Zn.
