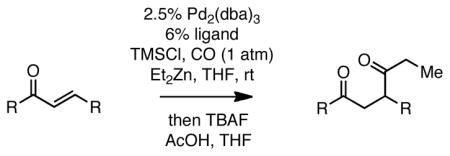
Table 2.
Carbonylative Conjugate Addition of Et2Zn To α,β-Unsaturated Ketones.a
 | |||
|---|---|---|---|
| entry | substrate | % yield | |
| L=PPh3 | L=PCy3 | ||
| 1 | 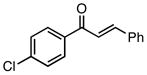 |
82 | 80 |
| 2 | 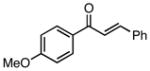 |
81 | 81 |
| 3 | 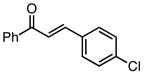 |
84 | 82 |
| 4 | 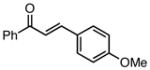 |
79 | 80 |
| 5 | 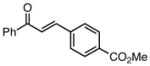 |
86 | 87 |
| 6 | 52 | 82 | |
| 7 | 67 | 66b | |
| 8 | 85 | 86 | |
| 9 | 56 | 48 | |
| 10 | 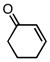 |
68 | 67 |
| 11 | 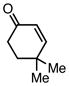 |
72 | 55c |
Reaction with 1.3 equiv Et2Zn, 2.3 equiv TMSCl, 1 atm CO, for 4 h (7 h for entry 4; 24 h for entry 11). Subsequent to reaction, the mixture was quenched at 0 °C with acetic acid (1.5 equiv) and TBAF (1.5 equiv) for 10 minutes and then treated with saturated Na2CO3.
Yield adjusted to account from unremovable impurity (ca. 10%).
10% Pd2(dba)3, 24% ligand, and 1.95 equiv Et2Zn employed.
