Table 3.
Catalytic Carbonylative Conjugate Addition of Et2Zn to α,β-Unsaturated Aldehydes.a
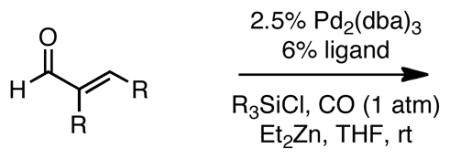 | |||||
|---|---|---|---|---|---|
| entry | substrate | product | R3SiCl | % yield | |
| L:PPh3 | L:PCy3 | ||||
| 1 | 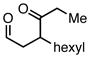 |
Et3SiCl | 78 | 69 | |
| 2 | 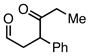 |
Et3SiCl | 41 | 39 | |
| 3 | 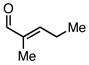 |
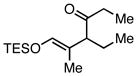 |
Et3SiCl | 90b | 72b |
| 4 | 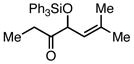 |
Ph3SiCl | 63 | 60 | |
| 5 | 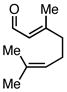 |
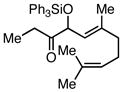 |
Ph3SiCl | 68c | |
| 6 | 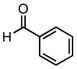 |
Et3SiCl | N.R. | ||
Reaction with 1.3 equiv Et2Zn, 2.3 equiv TMSCl, 1 atm CO; 4 h for entries 1 and 2; 20 h for entries 3 and 4; 24 h for entries 5 and 6. Subseqeunt to reaction, the mixture was quenched at 0 °C with acetic acid (1.5 equiv) and TBAF (1.5 equiv) for 10 minutes and then treated with saturated Na2CO3.
Quench (footnote a) was omitted from this reaction.
A 2:1 mixture of olefin isomers was obtained.
