Abstract
The goal of this paper was to investigate the amplitude and sub-100 Hz frequency content of surface electromyography (EMG) signals obtained from agonist, antagonist and synergist muscles during a heel-raise task sustained to failure. Twenty-two healthy adults, 14 men and 8 women participated in the study. Surface EMG data from the raising and lowering phases of the movement were studied in the time (EMG amplitude) and frequency (wavelet transform) domains. For the raising phase, we found a significant increase in the EMG amplitude of all muscles studied throughout the task (P < 0.02); however, for the lowering phase, we found a decrease in overall muscle activation for the medial gastrocnemius and tibialis anterior. Additionally, we found higher 13–30 and 30–50 Hz normalized power during the raising phase for the triceps surae prior to task failure and at task failure compared with the beginning and midway of the task (P < 0.05); during the lowering phase, however, we found higher normalized power from 30 to 50 Hz for the triceps surae (P < 0.01) and higher 13–30 Hz normalized power for the tibialis anterior (P < 0.01) at task failure compared with the beginning and midway of the task. Finally, we showed that a dynamic task performed until failure can induce different activation strategies for agonist, antagonist and synergist muscles, and that the frequency content below 100 Hz contains useful information about the neural activation of these muscles in relation to task failure that is not evident from the EMG amplitude.
Keywords: EMG, Oscillations, Wavelet transforms, Dynamic task, Beta band, Piper band
Introduction
Task failure is defined as the inability of an individual to keep performing a requested task. The mechanisms that cause task failure can be different from the mechanisms of muscle fatigue (Barry and Enoka 2007; Hunter et al. 2004). It is suggested, that the causes of task failure are task specific (Enoka and Duchateau 2008; Hunter et al. 2004). Nonetheless, most studies on task failure are focused on constant isometric contractions (Barry and Enoka 2007; Enoka and Stuart 1992; Enoka and Duchateau 2008; Hunter et al. 2004; Rudroff et al. 2007) and the mechanisms that contribute to task failure during dynamic tasks are not clear (Karlsson et al. 2000; Svantesson et al. 1998a, b).
During isometric and dynamic contractions task failure has been associated with the following neural mechanisms and EMG changes: (1) increased agonist muscles EMG activity, likely dominated by the recruitment of additional motor units and modest changes in discharge rate (constant isometric contraction; Hunter et al. 2004); (2) increased activation of synergist muscles (constant isometric contraction; Rudroff et al. 2007); (3) significant decreases in EMG mean power frequency in both raising and lowering phases of the movement (dynamic heel-raise test; Svantesson et al. 1998a). The authors, however, are not aware of any study that investigates the sub-100 Hz frequency content of EMG signals during tasks sustained until failure. There is evidence that during voluntary contractions, different frequency bands sub-100 Hz of the EMG signals may be associated with cortical drives to the motor neuron pool (Brown 2000; Neto and Christou 2010; Neto et al. 2010). Therefore, it is possible that the power below 100 Hz in the EMG signal may provide important information about task failure.
Analyzing recordings of the neural activation of muscles during dynamic contractions is challenging because EMG signals are non-stationary (Hostens et al. 2004). One way to address this issue is to use a time–frequency analysis technique (Balestra et al. 2001), such as wavelet transforms. Wavelet transforms deal with the non-stationarity of EMG signals obtained during dynamic contractions (Karlsson et al. 2000; Neto and Magini 2008; Neto and Marzullo 2009; So et al. 2009) because they analyze EMG signals in a very short duration (So et al. 2009) so that the change in timing and frequency content can be evaluated simultaneously (Felici 2006; Kouzaki et al. 2004). The frequency structure of EMG signals analyzed through time can present relevant physiological meaning and it has been previously used for assessing localized muscle fatigue during dynamic contractions (Bonato et al. 1996, 2001; Gandevia 2001).
During dynamics tasks muscles alternately perform lengthening (eccentric) and shortening contractions (concentric). It has been shown that the mean firing rate and the amplitude and frequency structure of EMG during eccentric and concentric phases of a movement can be different (Søgaard et al. 1996; Potvin 1997; Svantesson et al. 1998b; Christou et al. 2003; Hostens et al. 2004). However, the effects of fatigue on the EMG activity of muscles during eccentric and concentric phases of a continuous dynamic task seem to be similar in both the time (Svantesson et al. 1998a, b) and frequency domains (Potvin 1997).
The purpose of this study, therefore, was to investigate the amplitude and sub-100 Hz frequency content of surface EMG signals obtained from several muscles during the lowering and raising phases of a heel-raise task performed until failure. Our main hypotheses were an increase in the EMG amplitude and observable sub-100 Hz changes in the frequency structure of the EMG signals of the muscles involved in the task prior to and at task failure. We hypothesized that power sub-100 Hz in the EMG signal would change with time during the fatiguing dynamic task similarly for the lowering and raising phases of the task.
Methods
Subjects
Twenty-two healthy adults, 14 men and 8 women [age 21 ± 1 years; height 171 ± 2 cm; weight 65 ± 2 kg (mean ± SE)] volunteered to participate in the study. All the subjects were right handed with no known neuromuscular disorders and were naive to the experimental protocol and procedures. The study was approved by the local ethics committee (Universidade Iguaçu, UNIG) and conducted following the Helsinki protocol of ethical principles for medical research involving human subjects. All subjects gave informed written consent to participate in the study.
Dynamic fatigable task
Subjects were instructed to perform as many heel raises as possible at a pace controlled by a metronome configured as in Haber et al. (2004) to give 46 beats/min (23 raises/min); this pace was neither too fast nor too slow to compromise subjects' balance. We decided to study task failure during a standing heel-raise task because this task is a commonly employed clinical test for evaluating muscle function in the triceps surae (Svantesson et al. 1998a) and a reliable protocol and apparatus to standardize this task has been developed previously (Haber et al. 2004). During the task, the subjects maintained the knee of the testing leg fully extended and the knee of the contralateral leg positioned in flexion and suspended in air. The testing leg was chosen to be the dominant leg of the subjects. Task termination occurred based on the following two factors: (1) the pace could no longer be maintained in three sequential heel raises; (2) the predetermined height of movement could no longer be attained in three sequential heel-raise attempts. Throughout the entire task each subject was permitted to place one hand flat against a wall for balance. A research assistant was responsible to make sure that the subjects were not using the wall for purposes other than balancing themselves.
Standing heel-raise test apparatus
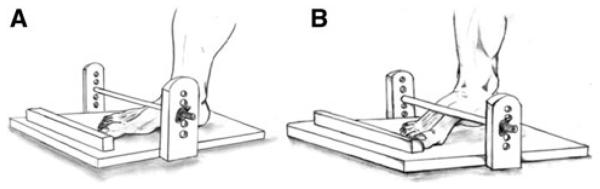
To establish a constant vertical displacement for all heel raises performed during the task before failure, we used a standing heel-raise apparatus (Haber et al. 2004; Fig. 1). The apparatus consists of a rod and a foot positioning device attached to a platform. Both the rod and foot positioning device are adjustable to allow subjects to perform a heel-raise task with a constant 5 cm heel vertical displacement, despite the differences in individuals' foot dimensions. The apparatus is adjusted to limit the height of each heel raise to 5 cm as follows: first, the subject stands barefooted on one leg with the knee fully extended; second, the subject raises the heel until the navicular bone touches the rod. The foot positioning device or the rod or both are adjusted to limit the height of each heel raise to 5 cm measured vertically from the platform floor to the heel.
Fig. 1.
The apparatus designed to standardize a standing heel raise test developed by Haber et al. (2004). The apparatus consists of a rod and a foot positioning device attached to a platform, both of which are adjustable. Before each trial, the apparatus is first adjusted to limit the height of each heel raise to 5 cm as follows: first, the subject stands barefooted on one leg with the knee fully extended (a); second, the subject raises the heel until the navicular bone touches the rod (b). The foot positioning device or the rod or both are adjusted to limit the height of each heel raise to 5 cm
Data acquisition
Surface EMG signals were obtained continuously throughout the task using an eight channel module (model EMG800C, EMG System, Brazil) with a total amplifier gain of 2000, a common mode rejection ratio of 120 dB, sampled at 2,000 Hz and band-pass filtered (20–500 Hz). A 12-bit converter digitalized the analog signals with a sampling frequency of anti-aliasing 2.0 kHz for each channel. Pre-amplified (×100) bipolar superficial electrodes of Ag/AgCl were used with inter-electrode (center-to-center) distance of 20 mm. After shaving and cleaning the skin with alcohol, the determination of the muscles and anatomical landmarks were done via palpation and the electrodes were placed over the medial and lateral gastrocnemius, medial portion of the soleus, tibialis anterior, and peroneus longus muscles according to SENIAM (surface EMG for a non-invasive assessment of muscles) procedures (Hermens et al. 2000) and guided by bone prominences and the direction of the muscle fibers.
To identify task failure by failing to maintain the metronome pace and to determine the beginning and ending of each heel raise and lowering cycle, a foot-switch (EMG System, Brazil) was placed in the point of heel contact of each subject and connected to the same eight channel module used to obtain the EMG recordings (model EMG800C, EMG System, Brazil). Footswitch recordings were sampled at 2,000 Hz and synchronized to the EMG recordings. Identification of task failure due to failing of maintaining the height of movement was done visually.
Data analysis
The coefficient of variation of the time taken to perform each cycle of the movement (raising and lowering parts) across subjects and times ranged from 10 to 15%. The average movement cycle was 2.6 s (due to the pace). Based on this knowledge, we were confident that the first second of each movement cycle was within the raising phase, whereas the last second was within the lowering phase. We also assumed that during the first second of the raising phase and the last second of the lowering phase the ankle performed similar range of motion. Footswitch recording were used to determine the first second (raising phase) and the last second (lowering phase) from each movement cycle. The first three heel-raise cycles were excluded from data analysis because subjects were adapting to the metronome. Then, for each subject, the total number of heel raises was divided into the following four time periods: the beginning of the task (beginning), midway through the task (midway), just prior to task failure (pre-failure), and at task failure (failure). Although at task failure subjects failed to keep either the height or the pace of the task, they still performed movement cycles longer than 2 s and, on average, achieved heights close to the target height. Thus, for consistency, data at task failure was analyzed the same way as data for the other time periods. Data analysis on each time period included three sequential heel raises cycles. Results were calculated considering 1 s of each phase of each cycle separate. The results obtained for three consecutive raising phases were averaged together and the results obtained for three consecutive lowering phases were also averaged together.
EMG data from the raising and lowering phases of the tasks were studied in the time (EMG amplitude) and frequency (wavelet transform) domains. For the time domain analysis we calculated the amplitude of each 1-s window of the EMG signals using the root mean square.
Wavelet analysis
The frequency domain analysis was done using Morlet wavelet transform. Morlet wavelet represents a set of functions with the form of small waves created by dilations and translations from a simple generator function (Eq. 1) which is called Morlet mother wavelet (Addison 2002).
| (1) |
where η is dimensionless time and w0 is dimensionless frequency (in this study we used w0 = 6, as suggested by Grinsted et al. 2004). The wavelet transform applies the wavelet function as a band-pass filter to the time series (Eq. 2).
| (2) |
where s represents the dilation parameter (scale shifting), τ represents the location parameter (time shifting) and the basic function ψs,τ(t) is obtained by dilating and translating the mother wavelet ψ0(t) (Addison 2002). For the w0 = 6 Morlet wavelet the scale is almost equal to the Fourier period (Fourier period = 1.03 s).
The importance of wavelet transform to the analysis of EMG signals obtained during dynamic tasks is that the wavelet transform can detect if there are any transient events that take place within a chosen window of time by determining how the amplitude versus frequency characteristics of the signal changes with time (Zazula et al. 2004).
In this paper wavelet transforms were calculated using a matlab function developed by Torrence and Compo (1998) (available at http://paos.colorado.edu/research/wavelets). An algorithm was developed in Matlab 7.0.1 (MathWorks Inc.) to measure the relative importance of different frequency bands of the interference EMG signals (please see next section for more details). We defined the normalized wavelet scale-averaged power spectrum (NWPS, Fig. 4) as the squared weighted modulus of the wavelet transform normalized by the sum of the squared weighted modulus over all scales for each instant to time (Eq. 3).
| (3) |
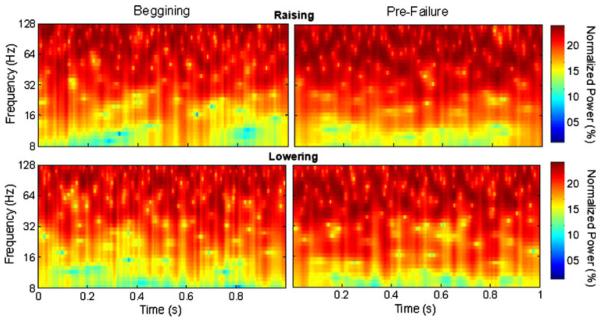
Fig. 4.
Examples of the normalized wavelet spectra calculated from the raising phase (top row) and lowering phase (bottom row) of the beginning (left column) and pre-failure (right column) periods of the task for the lateral gastrocnemius; spectra were calculated from the data shown in Fig. 2 (second row)
The normalized wavelet scale-averaged power spectrum (hereafter referred to as the normalized wavelet spectrum) shows the relative importance through time of each scale (can be related to a frequency) of the signal with intensities ranging from 0–100%. Thus, the normalized wavelet spectrum can be used to compare the strength of different EMG oscillations within the same signal at different times, or between two different signals.
Normalized wavelet spectra were plotted for each 1-s window obtained from the raising and lowering phases of the movement. Because the bands of interest were active during the whole duration of the analyzed windows, the relative importance of each frequency band for each 1-s window (2,000 points) was estimated as a percentage of the power within the band and the total power of the signal in each window (e.g. Eq. 4 shows how the normalized power of the 13–30 Hz frequency band [Beta Band normalized power (BBNP)] was estimated).
| (4) |
Interference EMG and bands of interest
During voluntary contractions specific frequency bands of the EMG signals have been associated with cortical drives to the motor neuron pool. The most often studied frequencies bands are below 100 Hz, as higher frequencies seem to be related to the shape of the action potentials and does not reflect carrying frequencies of the EMG signal (Myers et al. 2003). We determined the importance of three frequency bands 13–30 Hz (beta drive), 30–50 Hz (low-gamma drive) and 50–100 Hz (high gamma drive) because they have been previously associated with specific cortical drives (Brown 2000; Neto and Christou 2010) and associated with changes in voluntary effort (Neto et al. 2010). Interference, instead of rectified, EMG signals were used in this study because they provide accurate information about the oscillatory input to EMG signals (Neto et al. 2010; Neto and Christou 2010).
Statistics
A three-way analysis of variance (2 phases × 5 muscles × 4 times) with repeated measures on all factors was used to compare the EMG amplitude. A four-way analysis of variance (2 phases × 5 muscles × 4 times × 3 frequency bands) with repeated measures on all factors was used to compare the 13–30, 30–50 and 50–100 Hz normalized power. Significant ANOVA results were followed by appropriate post hoc tests with Bonferroni corrections. A significance level of P < 0.05 was used to identify statistical significance. For clarity, some graphs are presented as the average from the three agonist muscles (triceps surae). Data are reported as means ± SE (standard error). All statistical procedures were carried out using SPSS v.17.0 (SPSS Inc., Chicago, IL).
Results
The task mean duration was 194 ± 120 s, during which subjects performed an average of 73 ± 42 heel raises before task failure. Figure 2 shows an example of the raw EMG recordings from each muscle at the beginning of the task and prior to task failure.
Fig. 2.
Raw EMG signal collected from the medial gastrocnemius (GM), lateral gastrocnemius (LG), soleus (SOL), peroneus longus (PL) and tibialis anterior (TA) during three raising and lowering movement cycles in the beginning of the task and prior to task failure. Markers in the second movement cycle of the beginning of the task for the GM muscle illustrate the 1-s periods analyzed for the raising and lowering phases of the movement
EMG amplitude
We found a significant main effect for phase (F1,21 = 68.45, P < 0.01). For all muscles and times, EMG mean amplitude during the raising phase was significantly (P < 0.01) higher than during the lowering phase. We also found significant main effects for muscles (F4,84 = 31.15, P < 0.01) and times (F3,63 = 14.13, P < 0.01). The comparison of EMG amplitude across muscles demonstrated that the tibialis anterior exhibited lower values than all other muscles throughout the whole task (P < 0.01). No difference was found between the amplitude of the triceps surae and peroneus longus muscles (P > 0.2). No consistent results for the different times were found for both movement phases and across all muscles.
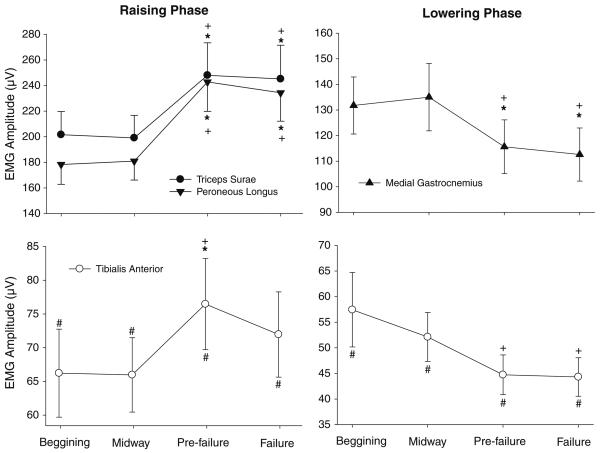
We found a significant phase × muscle × time interaction for the EMG amplitude (F12,252 = 6.54, P < 0.01). Post hoc tests demonstrated that for the raising phase, there was an increase in EMG amplitude for all studied muscles (P < 0.02) (Fig. 3; left column). The triceps surae and the peroneus muscles demonstrated higher EMG activity during pre-failure and at task failure when compared with midway through the task and to the beginning of the task (P < 0.01; Fig. 3, top left). Additionally, the tibialis anterior muscle demonstrated higher EMG amplitude prior to task failure than during the beginning and midway of the task (P < 0.01; Fig. 3, bottom left). In contrast, during the lowering phase there was a significant (P < 0.01) decrease in amplitude for the medial gastrocnemius and tibialis anterior with time (Fig. 3, right column). The medial gastrocnemius muscles demonstrated lesser EMG amplitude prior to task failure and at task failure compared with the beginning and midway through the task (P < 0.01; Fig. 3, top right). Additionally, the tibialis anterior muscle demonstrated lower EMG amplitude prior to task failure than midway through the task (P < 0.01; Fig. 3, bottom right).
Fig. 3.
Mean amplitude of the EMG signals of the triceps surae, tibialis anterior, and peroneus longus muscles at the beginning, midway, pre-failure and task failure divided in raising phase (left column) and lowering phase (right column). Only muscles that exhibited significant changes with time are shown. Asterisk different from “beginning”; cross different from “midway through”; hash different from other muscles
EMG frequency structure
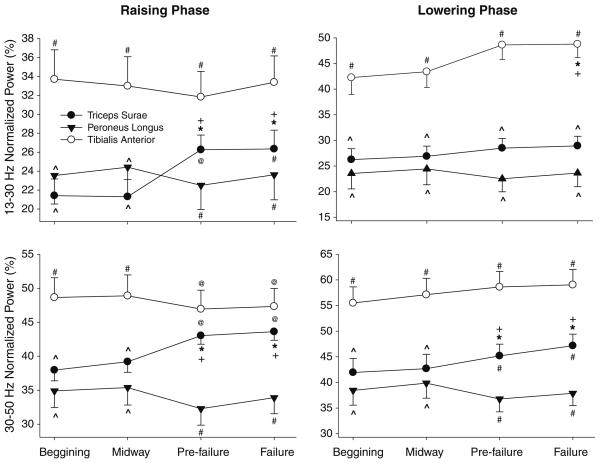
Figure 4 shows an example of a normalized wavelet spectrum of a lateral gastrocnemius EMG signal obtained from the raising phase (top row) and lowering phase (bottom row) of the first cycle of the beginning and pre-failure periods of the task (calculated from data exemplified in Fig. 2, second row). The overall results demonstrated that there was a significant phase × muscle × time × frequency bands interaction for the wavelet normalized power (F24, 504 = 4.15, P < 0.01). Post hoc tests demonstrated that during the raising phase (Fig. 5, left column) there was a progressive amplification of the triceps surae muscles EMG oscillations from 13 to 30 and 30 to 50 Hz throughout the task. Both the medial and lateral gastrocnemius muscles demonstrated significant higher values of 13–30 and 30–50 Hz normalized power prior to task failure and at task failure when compared with the beginning and midway of the task (P < 0.01; Fig. 5, left column). The normalized power from 30 to 50 Hz of the lateral gastrocnemius was also significantly greater midway through the task when compared with the beginning of the task (P < 0.01). Additionally, 30–50 Hz normalized power of soleus muscle was significantly greater prior to task failure and at task failure when compared with the beginning and midway of the task (P < 0.05). During the lowering phase the effects of fatigue on the 13–30 Hz frequency band of the EMG signals were different than during the raising phase (Fig. 5, top right). An increase in 13–30 Hz normalized power at task failure when compared with the beginning and midway of the task was observed only for the tibialis anterior (P < 0.01; Fig. 5, top right). On the other hand, the changes in the 30–50 Hz normalized power with time were very similar for every muscle between the lowering and raising phases. During the lowering phase, the medial gastrocnemius muscle demonstrated higher values 30-50 Hz normalized power prior to task failure and at task failure when compared with the beginning and midway of the task (P < 0.01; Fig. 5, bottom right). In addition, the lateral gastrocnemius muscles demonstrated higher values 30–50 Hz normalized at task failure when compared with the beginning and midway of the task (P < 0.01; Fig. 5, bottom right). No consistent significant changes with time were found for the 50–100 Hz frequency band for all muscles studied (P > 0.3).
Fig. 5.
13–30 Hz (top row) and 30–50 Hz (bottom row) normalized power of the triceps surae, peroneus longus and tibialis anterior muscles divided in raising phase (left column) and lowering phase (right column). Asterisks different from “beginning”; cross different from “midway through”; hash different from other muscles; ^different from tibialis anterior; @ different from peroneus longus
The comparison of 13–30 and 30–50 Hz normalized power across muscles demonstrated that the tibialis anterior, for the most part, exhibited higher values than all other muscles throughout the task (P < 0.02). The exceptions were during the raising phase for the 30–50 Hz normalized power when the tibialis anterior and the triceps surae muscles were not different during pre-failure and failure (P > 0.17). The 13–30 Hz normalized power was significantly greater for the triceps surae muscles during the raising phase compared with the peroneus longus prior to task failure and at task failure (P < 0.01). Finally, the 30–50 Hz normalized power was significantly greater for the triceps surae compared with the peroneus longus prior to task failure and at task failure for both the lowering and raising phases of the task (P < 0.01).
Discussion
The aim of this study was to investigate the amplitude and sub-100 Hz frequency content of the neural activation of agonist, antagonist, and synergist muscles during the raising and lowering phases of a dynamic task sustained to failure. Our findings demonstrate the following: (1) In contrast to previous studies, the changes observed in the amplitude and frequency characteristics of the EMG during the task were different for the lowering and raising phases of the task; (2) contractions performed till task failure during a dynamic task can induce different activation strategies for agonist, antagonist and synergist muscles.
Lowering versus raising phase
We found that the EMG amplitude was higher during the raising phase than during the lowering phase of the movement (Potvin 1997; Svantesson et al. 1998a, b; Christou et al. 2003). The reasons for the greater neuro-muscular activation during the raising phase were probably elevated motor unit discharge rates and increased motor unit recruitment as the triceps surae muscles were required to produce force to overcome gravity (Søgaard et al. 1996) and compensate for the different region on the force–velocity curve (Pasquet et al. 2006).
Our results contradict our hypothesis that the changes observed in the amplitude and frequency characteristics of the EMG during the task would be similar for the lowering and raising phases of the task (Potvin 1997; Svantesson et al. 1998a, b). For the raising phase of the movement, we found higher EMG amplitude of all muscles involved in the task prior to task failure and at task failure compared with the beginning and midway through the task (Fig. 3, left column). During the lowering phase, however, the medial gastrocnemius and tibialis anterior exhibited lower EMG amplitude prior to task failure and at task failure compared with the beginning and midway through the task (Fig. 3, right column). For the raising phase of the movement, we found higher normalized power from 13–30 and 30–50 Hz for the triceps surae muscles prior to task failure and at task failure compared with the beginning and midway through the task (Fig. 5, left column). During the lowering phase, however, we found higher 30–50 Hz normalized power for the triceps surae and higher 13–30 Hz normalized power for the tibialis anterior at task failure compared with the beginning and midway through the task (Fig. 5, right column).
Raising phase
During the raising phase of the movement, we observed a significant increase in the amplitude of the EMG signals of all muscles studied during the task (Fig. 3). Increases in surface EMG amplitude of agonist muscles during tasks performed until failure have been largely observed before and are normally attributed to increased descending drive to the motor neuron pool (Hunter et al. 2002, 2008; Mottram et al. 2006). Interestingly, our results demonstrated that the increase in EMG amplitude was not the same for all frequency bands of the signal (see next section).
We found a significant increase with time in the EMG amplitude of the peroneus longus (Fig. 3), a synergist muscle in the raising phase of the task. Increments of synergist muscle activity have been described in fatiguing isometric tasks (Maluf et al. 2005; Kouzaki and Shinohara 2006; Rudroff et al. 2007; Sirin and Patla 1987) and have been associated with possible longer (Kouzaki and Shinohara 2006; Sirin and Patla 1987) and briefer times to failure (Rudroff et al. 2007). It is unclear what the increase of synergist muscle activity caused in our protocol.
Our results also showed a significant slight increase in the activation of the tibialis anterior (antagonist muscle of the raising phase) muscle for the raise phase of the task during pre-failure (Fig. 3, bottom left). This supports previous studies that show an increase in antagonist EMG activity during fatiguing contractions (Psek and Cafarelli 1993; Rothmuller and Cafarelli 1995; Weir et al. 1998), but at the same time contrast findings by Hassani et al. (2006). The fact that at task failure the tibialis anterior EMG amplitude was not significantly different than during the beginning of the task suggests that, although the activation of this muscle increased slightly during the task, co-activation was not the cause of task failure. Other studies have shown that co-activation does not appear to be the cause of task failure in isometric sub-maximal position and force tasks (Hunter et al. 2008; Maluf et al. 2005).
From our frequency domain analysis, our results demonstrated significantly stronger 13–30 and 30–50 Hz oscillations at task failure compared with the beginning of the task for the medial and lateral gastrocnemius (Fig. 5, left column). This was not the case for the 50–100 Hz oscillations, however. It is possible that the changes observed in power from 13–50 Hz on the surface EMG were associated to an increase in the variability of motor unit interspike intervals due to fatigue (Garland et al. 1994). Furthermore, it has been shown that beta band oscillations on single motor unit EMG data are correlated to synchronization of action potential discharge times (Farmer et al. 1997; Moritz et al. 2005; Christou et al. 2007; Lowery et al. 2007). Additionally, it has been suggested that the effects of changes in firing rate and synchronization are reflected on the EMG spectrum at frequencies in the neighborhood of 15–25 Hz (DeLuca 1997). The stronger drive found from 13 to 30 may suggest that synchrony increased prior to task failure and at task failure (Gabriel et al. 2001). EMG signals in the 30–50 Hz frequency range have also been broadly associated with specific cortical drives to the muscle (Salenius et al. 1996; Brown 2000). Additionally, it has been suggested that adequate processing of sensory information may require cyclical brain events in the 30–50 Hz range (Galambos et al. 1981) and that muscular firing patterns in these frequencies likely reflect communication between the sensorimotor cortex and motor units (Salenius et al. 1996). Thus, the increase in strength of the 30–50 Hz frequency band prior and at task failure may be associated with changes in sensory feedback that occur due to fatigue near task failure (Hunter et al. 2008; Taylor and Gandevia 2008). More studies should be done in order to better understand the meaning of these oscillations found in surface EMG signals.
Lowering phase
We observed a significant decrease during the task in the amplitude of the medial gastrocnemius and tibialis anterior EMG signals obtained during the lowering phase (Fig. 3, right column). The activation of the medial gastrocnemius and tibialis anterior during the lowering phase is a mechanism to control the speed of movement of the descending body and it is important to keep the pace of the task. We speculated that with time subjects decreased the descending command to both muscles during the lowering phase attempting to keep the pace and save energy, as these muscles were increasingly being activated during the raising phase.
During the lowering phase we found an increase at task failure, not found during the raising phase, in the 13–30 Hz normalized power of the tibialis anterior (Fig. 5, top right). The reason for this increase is not clear, but it shows that the relative contribution of the oscillations between 13 and 30 Hz in the EMG signal can be different between eccentric and concentric phases of dynamic cyclic tasks.
Additionally, during the lowering phase, we found an increase in 30–50 Hz normalized power of the triceps surae during the task (Fig. 4, bottom right). As it can be seen in Fig. 5 (bottom row) the 30–50 Hz activation was very similar during the lowering and raising phases of the movement. These results corroborate with the theory that the 30–50 Hz band may be related to processing of sensory information and communication between the sensorimotor cortex and motor units (Galambos et al. 1981; Salenius et al. 1996) that were probably similar between the two phases of the movement.
Activation of agonist, antagonist and synergist muscles
We found no significant differences in time in the activation of the tibialis anterior (antagonist of the raising phase) during the raising phase of the task. However, when we compared the results of 13–30 and 30–50 Hz normalized power, across the different muscles, we found that, for the most part, the tibialis anterior presented higher 13–50 Hz normalized power values than all other muscles throughout the task. The reason for these results is unclear; one possibility is that the central nervous system activated antagonist muscles differently than agonist and synergist muscles, which has been shown to be true for constant isometric contractions (Lévénez et al. 2005).
Another interesting finding is that although the triceps surae and the peroneus longus (synergist muscle of the raising phase) exhibited no difference in EMG amplitude throughout the task, they exhibited different frequency structure through time. While the 13–30 and 30–50 Hz normalized power were not different between the triceps surae and the peroneus longus muscles in the beginning of the task, the activation in this frequency bands was significantly different between these muscles prior to task failure and at task failure (Fig. 5, left column). These results demonstrate that the neural strategy to activate agonist and synergist muscles changed during the task and that these differences in neural strategy cannot be inferred by the amplitude of the EMG signals.
Although the differences found between the EMG frequency structure of the agonist, antagonist and synergist muscles are clear, it is important to consider that there are other factors other than the activation of the motor units that can alter the frequency domain characteristics of surface EMG signals. For instance, the spectral properties of the surface action potentials are strongly influenced by the distance between the active muscle fibers and the EMG detection point; the recruitment of additional motor units, for example, may contribute to either the high- or low-frequency bands of the surface EMG signals depending on the depth of the motor unit (Farina 2006). Additionally, the shortening of muscle fibers during a dynamic contraction and the effects of fatigue in the intrinsic properties of the muscle can cause changes in the conduction velocity of muscle fibers altering the shape of action potentials, and consequently altering the EMG frequency structure (Farina et al. 2004). Changes on action potential shape, however, will most likely cause changes in the EMG power spectrum between 60 and 150 Hz (Farina et al. 2004). Thus, the results obtained for normalized power of the 13–30 and 30–50 Hz oscillations are promising and more studies should be done in order to better understand the meaning of these oscillations found in surface EMG signals.
Conclusion
Most task failure studies investigate constant isometric contractions. The purpose of this study was to determine the amplitude and frequency structure of the EMG activity of muscles involved in a dynamic task. We provide novel evidence that the frequency content below 100 Hz contains useful information about the neural activation of the muscles that is not evident from the EMG amplitude. In addition, we demonstrate that frequency bands associated with neural drives to the muscles were stronger prior to and at task failure. Finally, we have shown that during dynamic task performed until failure muscle activation changes differently for the raising and lowering phases of the movement and that agonist, antagonist, and synergist muscles were differently activated prior to task failure and during task failure.
Acknowledgments
This work was supported by National Institute on Aging Grant R01AG-031769 to E.A. Christou. The authors would like to thank Prof. Evangelos Christou for valuable suggestions.
References
- Addison PS. The illustrated wavelet transform handbook. Taylor and Francis Group; New York: 2002. [Google Scholar]
- Balestra G, Frassinelli S, Knaflitz M, Molinari F. Time– frequency analysis of surface myoelectric signals during athletic movement. IEEE Eng Med Biol Mag. 2001;20:106–115. doi: 10.1109/51.982282. [DOI] [PubMed] [Google Scholar]
- Barry BK, Enoka RM. The neurobiology of muscle fatigue:15 years later. Integr Comp Biol. 2007;47:465–473. doi: 10.1093/icb/icm047. [DOI] [PubMed] [Google Scholar]
- Bonato P, Gagliati G, Knaflitz M. Analysis of myoelectric signals recorded during dynamic contractions: a time–frequency approach to assessing muscle fatigue. IEEE Eng Med Biol Mag. 1996;15:102–111. [Google Scholar]
- Bonato P, Cheng MSS, Gonzalez–Cueto J, Leardini A, O'Connor J, Roy SH. EMG-based measures of fatigue during a repetitive squat exercise: assessment of dynamic conditions can provide information about compensatory muscle function in ACL patients. IEEE Eng Med Biol Mag. 2001;20:133–143. doi: 10.1109/51.982285. [DOI] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Christou EA, Shinohara M, Enoka RM. Fluctuations in acceleration during voluntary contractions lead to greater impairment of movement accuracy in old adults. J Appl Physiol. 2003;95(1):373–384. doi: 10.1152/japplphysiol.00060.2003. [DOI] [PubMed] [Google Scholar]
- Christou EA, Rudroff T, Enoka JA, Meyer F, Enoka RM. Discharge rate during low-force isometric contractions influences motor unit coherence below 15 Hz but not motor unit synchronization. Exp Brain Res. 2007;178:285–295. doi: 10.1007/s00221-006-0739-5. [DOI] [PubMed] [Google Scholar]
- DeLuca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163. [Google Scholar]
- Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586:11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Farina D. Interpretation of the surface electromyogram in dynamic contractions. Exerc Sport Sci Rev. 2006;34(3):121–127. doi: 10.1249/00003677-200607000-00006. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applications of cross-correlation methodologies to human motor unit recording. J Neurosci Methods. 1997;74:175–187. doi: 10.1016/s0165-0270(97)02248-6. [DOI] [PubMed] [Google Scholar]
- Felici F. Neuromuscular responses to exercise investigated through surface EMG. J Electromyogr Kinesiol. 2006;16:578–585. doi: 10.1016/j.jelekin.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Gabriel DA, Basford JR, Na KN. Neural adaptations to fatigue: implications for muscle strength and training. Med Sci Sports Exerc. 2001;33:1354–1360. doi: 10.1097/00005768-200108000-00017. [DOI] [PubMed] [Google Scholar]
- Galambos R, Makeig S, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci USA. 1981;78(4):2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol. 1994;76:2411–2419. doi: 10.1152/jappl.1994.76.6.2411. [DOI] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes Geophys. 2004;11:561–566. [Google Scholar]
- Haber M, Golan E, Azoulay L, Kahn SR, Shrier I. Reliability of a device measuring triceps surae muscle fatigability. Br J Sports Med. 2004;38:163–167. doi: 10.1136/bjsm.2002.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani A, Patikas D, Bassa E, Hatzikotoulas K, Kellis E, Kotzamanidis C. Agonist and antagonist muscle activation during maximal and submaximal isokinetic fatigue tests of the knee extensors. J Electromyogr Kinesiol. 2006;16:661–668. doi: 10.1016/j.jelekin.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Hostens L, Seghers J, Spaepen A, Ramon H. Validation of the wavelet spectral estimation technique in biceps brachii and brachioradialis fatigue assessment during prolonged low-level static and dynamic contractions. J Electromyogr Kinesiol. 2004;14:205–215. doi: 10.1016/S1050-6411(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Ryan DL, Ortega JD, Enoka EM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol. 2002;88:3087–3096. doi: 10.1152/jn.00232.2002. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Duchateau J, Enoka RM. Muscle fatigue and the mechanisms of task failure. Exerc Sport Sci Rev. 2004;32:44–49. doi: 10.1097/00003677-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Yoon T, Farinella J, Griffith EE, Ng AV. Time to task failure and muscle activation vary with load type for a submaximal fatiguing contraction with the lower leg. J Appl Physiol. 2008;105:463–472. doi: 10.1152/japplphysiol.90398.2008. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Yu J, Akay M. Time–frequency analysis of myoelectric signals during dynamic contractions: a comparative study. IEEE Trans Biomed Eng. 2000;47:228–238. doi: 10.1109/10.821766. [DOI] [PubMed] [Google Scholar]
- Kouzaki M, Shinohara M. The frequency of alternate muscle activity is associated with the attenuation in muscle fatigue. J Appl Physiol. 2006;101:715–720. doi: 10.1152/japplphysiol.01309.2005. [DOI] [PubMed] [Google Scholar]
- Kouzaki M, Shinohara M, Masani K, Fukunaga T. Force fluctuations are modulated by alternate muscle activity of knee extensor synergists during low-level sustained contraction. J Appl Physiol. 2004;97:2121–2131. doi: 10.1152/japplphysiol.00418.2004. [DOI] [PubMed] [Google Scholar]
- Lévénez M, Kotzamanidis C, Carpentier A, Duchateau J. Spinal reflexes and coactivation of ankle muscles during a submaximal fatiguing contraction. J Appl Physiol. 2005;99(3):1182–1188. doi: 10.1152/japplphysiol.00284.2005. [DOI] [PubMed] [Google Scholar]
- Lowery MM, Myers LJ, Erim Z. Coherence between motor unit discharges in response to shared neural inputs. J Neurosci Methods. 2007;163:384–391. doi: 10.1016/j.jneumeth.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Maluf KS, Shinohara M, Stephenson JL, Enoka RM. Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res. 2005;167:165–177. doi: 10.1007/s00221-005-0017-y. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Christou EA, Meyer FG, Enoka RM. Coherence at 16–32 Hz can be caused by short-term synchrony of motor units. J Neurophysiol. 2005;94:105–118. doi: 10.1152/jn.01179.2004. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Hunter SK, Rochette L, Anderson LK, Enoka RM. Time to task failure varies with the gain of the feedback signal for women, but not for men. Exp Brain Res. 2006;174:575–587. doi: 10.1007/s00221-006-0498-3. [DOI] [PubMed] [Google Scholar]
- Myers LJ, Lowery M, O'Malley M, Vaughan CL, Heneghan C, Clair Gibson A, Harley YX, Sreenivasan R. Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J Neurosci Methods. 2003;124:157–165. doi: 10.1016/s0165-0270(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Neto OP, Christou EA. Rectification of the EMG signal impairs the identification of oscillatory input to the muscle. J Neurophysiol. 2010;103:1093–1103. doi: 10.1152/jn.00792.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto OP, Magini M. Electromiographic and kinematic characteristics of Kung Fu Yau-Man palm strike. J Electromyogr Kinesiol. 2008;18:1047–1052. doi: 10.1016/j.jelekin.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Neto OP, Marzullo ACD. Wavelet transform analysis of electromyography Kung Fu strikes data. J Sports Sci Med. 2009;8(CSSI 3):25–28. [PMC free article] [PubMed] [Google Scholar]
- Neto OP, Baweja HS, Christou EA. Increased voluntary drive is associated with changes in the common oscillations from 13–60 Hz of interference but not rectified electromyography. Muscle Nerve. 2010 doi: 10.1002/mus.21687. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J. Specific modulation of motor unit discharge for a similar change in fascicle length during shortening and lengthening contractions in humans. J Physiol. 2006;577(2):753–765. doi: 10.1113/jphysiol.2006.117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin JR. Effects of muscle kinematics on surface EMG amplitude and frequency during fatiguing dynamic contractions. J Appl Physiol. 1997;82:144–151. doi: 10.1152/jappl.1997.82.1.144. [DOI] [PubMed] [Google Scholar]
- Psek JA, Cafarelli E. Behavior of coactive muscles during fatigue. J Appl Physiol. 1993;74:170–175. doi: 10.1152/jappl.1993.74.1.170. [DOI] [PubMed] [Google Scholar]
- Rothmuller C, Cafarelli E. Effect of vibration on antagonist muscle coactivation during progressive fatigue in humans. J Physiol. 1995;485:857–864. doi: 10.1113/jphysiol.1995.sp020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudroff T, Barry BK, Stone AL, Barry CJ, Enoka RM. Accessory muscle activity contributes to the variation in time to task failure for different arm postures and loads. J Appl Physiol. 2007;102(3):1000–1006. doi: 10.1152/japplphysiol.00564.2006. [DOI] [PubMed] [Google Scholar]
- Salenius S, Salmelin R, Neuper C, Pfurtscheller G, Hari R. Human cortical 40 Hz rhythm is closely related to EMG rhythmicity. Neurosci Lett. 1996;213:75–78. doi: 10.1016/0304-3940(96)12796-8. [DOI] [PubMed] [Google Scholar]
- Sirin AV, Patla AE. Myoelectric changes in the triceps surae muscles under sustained contractions. Evidence for synergism. Eur J Appl Physiol. 1987;56:238–244. doi: 10.1007/BF00640651. [DOI] [PubMed] [Google Scholar]
- So RCH, Ng JKF, Lam RWK, Lo CKK, Ng GYF. EMG wavelet analysis of quadriceps muscle during repeated knee extension movement. Med Sci Sports Exerc. 2009;41:788–796. doi: 10.1249/MSS.0b013e31818cb4d0. [DOI] [PubMed] [Google Scholar]
- Søgaard K, Christensen H, Jensen BR, Finsen L, Sjøgaard G. Motor control and kinetics during low level concentric and eccentric contractions in man. Electroenceph Clin Neurophysiol. 1996;101:453–460. [PubMed] [Google Scholar]
- Svantesson U, Osterberg U, Thomee R, Grimby G. Muscle fatigue in a standing heel-rise test. Scand J Rehab Med. 1998a;30:67–72. [PubMed] [Google Scholar]
- Svantesson U, Osterberg U, Thomee R, Peeters M, Grimby G. Fatigue during repeated eccentric–concentric and pure concentric muscle actions of the plantar flexors. Clin Biomech. 1998b;13(4–5):336–343. doi: 10.1016/s0268-0033(98)00099-0. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. 2008;104:542–550. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. A practical guide to wavelet analysis. B Am Meteorol Soc. 1998;79:61–78. [Google Scholar]
- Weir JP, Keefe DA, Eaton JF, Augustine RT, Tobin DM. Effect of fatigue on hamstring coactivation during isokinetic knee extensions. Eur J Appl Physiol Occup Physiol. 1998;78:555–559. doi: 10.1007/s004210050460. [DOI] [PubMed] [Google Scholar]
- Zazula D, Karlsson S, Doncarli C. Advanced signal processing techniques. In: Merletti R, Parker P, editors. Electromyography: physiology, engineering, and noninvasive applications. Wiley and sons; New Jersey: 2004. pp. 259–304. [Google Scholar]